Change in Internal Energy
- We know that the kinetic energy of a gas is proportional to the absolute temperature of the gas.
- The total kinetic energy of its particles can be given as:
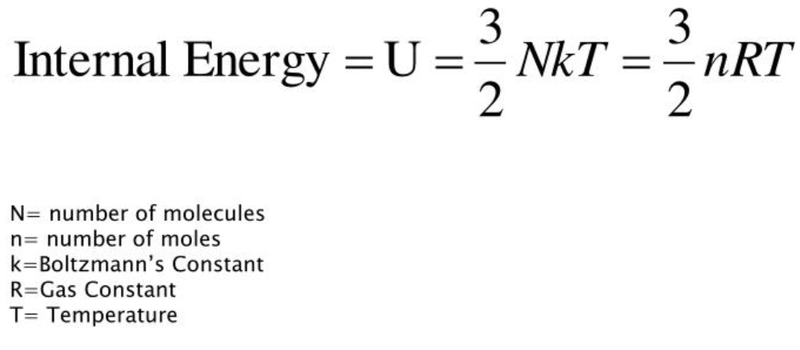
- Remember that in the formula above the gas is considered to have no potential energy between its molecules.
- Since Ideal gases have zero potential energy (due to negligible intermolecular forces), this formula yields the internal energy of an ideal gas.
The First Law of Thermodynamics
- The first law of thermodynamics states that energy is always conserved.

- Here work done by the gas is described by the formula: W = PΔV.
The Four Thermodynamic Processes
- The first law of thermodynamics can be used to describe the four thermodynamic processes.
- These processes are all obtained by keeping one variable fixed and their name alludes to the one being kept constant.
- The processes are:
- Isovolumetric
- Volume, V, is kept constant.
- This is also sometimes called an isochoric process.
- Isobaric
- Pressure, P, is kept constant
- If this one is confusing, think of the unit bars, to measure atmospheric pressure!
- Isothermal
- Temperature, T, is kept constant.
- Adiabatic
- Thermal energy, Q, is kept constant.
- This means that ΔU + W = 0
- Isovolumetric
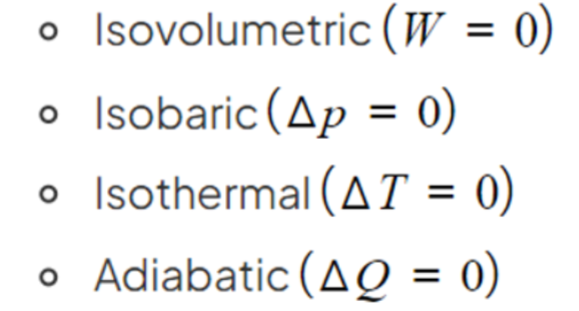
Graphing the Four Thermodynamic Processes
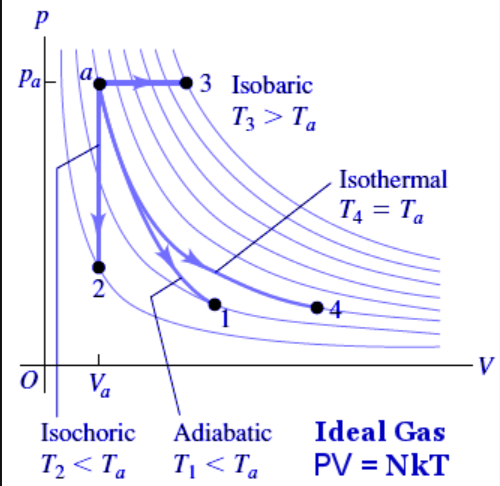
- The different curving lines represent how the gas behaves at different temperatures, while the y-axis is for pressure and the V axis is for volume.
- Isovolumetric/isochoric processes are a vertical movement.
- Isobaric processes are a horizontal movement.
- Isothermal processes are along one of the temperature lines.
- Adiabatic processes shift from one line to another.
- You can tell an adiabatic process apart from temperature lines due to them having a steeper gradient.
Adiabatic Expansion
- An adiabatic process is one in which there is no exchange of heat between the system and the surroundings.
- If we wanted to increase the volume without adding heat, then the internal energy must go down, resulting in a decrease in temperature.
- Likewise, to decrease the volume without adding any heat, internal energy must increase to that the thermal energy is still equal to zero, resulting in an increase in temperature.

- An adiabatic expansion can be defined by the following equation:
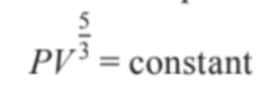
- We can see that P and V^5/3 are inversely proportional to one another.
- Therefore, the shape of the curve of adiabatic expansion can be given as:
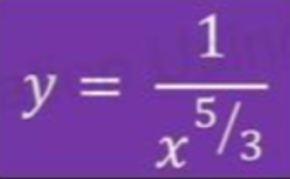
Sources