What is Thermal Radiation?
- Thermal radiation is one of the main ways of transferring thermal energy, alongside convection and conduction.
- Thermal radiation is the transfer of energy by electromagnetic waves.
- Unlike conduction and convection, it does not need particles to travel and can transfer energy through a vacuum.
- Certain surfaces absorb and emit thermal radiation much more effectively.
- That's why a black car heats up much quicker than a white car on a hot day.
- Any surface which is a good absorber of radiation will also be a good emitter.
- Black surfaces emit and absorb radiation well while white surfaces are poor at absorbing and emitting.
Black Bodies
- A Black body is defined as a body that emits all the radiation incident upon it in the same pattern of wavelengths.
Black-body Radiation
- Solids can absorb many more wavelengths than atmospheric gases.
- Depending on the color of a solid, it is possible to determine what wavelengths it cannot absorb.
- This is because the wavelengths that aren't absorbed are reflected, and we can see them!
- For example, a green object reflects green light, which makes it appear green to us.
- It absorbs all non-green wavelenghts.
- A black object absorbs all wavelengths.
- Likewise, a white object doesn't absorb any wavelengths and reflects them all back instead.
Luminosity
- Luminosity is defined as the total amount of radiation a black body emits per second.
- In essence, luminosity defines power, as it is also calculated as energy over time.
- Luminosity can also be calculated using the Stefan-Boltzmann equation:
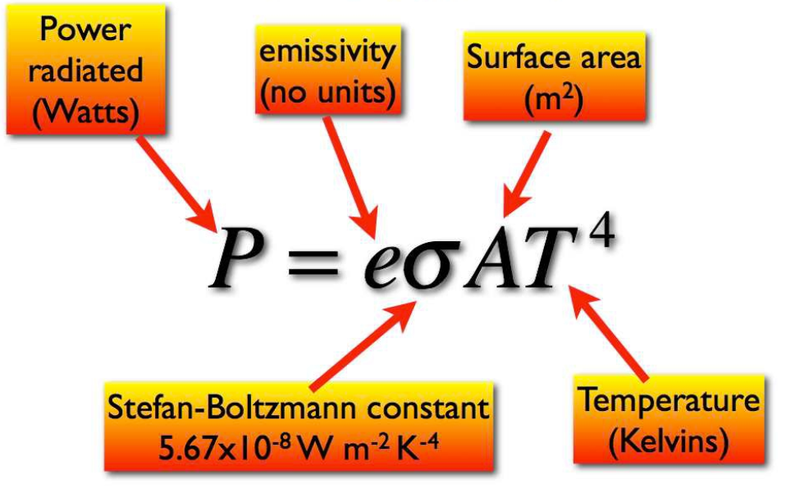
- Luminosity is often written as L, or P.
- For a spherical body, the luminosity is calculated as:

- As a black body emits as much power as it absorbs, the Stefan-Boltzmann law works for both emission and absorption problems involving black bodies.
Emissivity
- In reality, not all black bodies are perfect radiators.
- This means that they don't emit the same energy they absorb.
- This means the definition of emissivity is needed.
- Emissivity is defined as the ratio of energy radiated by a body compared to the theoretical energy a perfect black body at the same temperature would emit.
- Thus by definition the emissivity of a perfect black body is 1.
The Black Body Spectrum
- All objects emit radiation of some kind.
- The "color" of that radiation depends on the object's temperature.
- Imagine a yellow light bulb giving out light.
- It emits some radiation at some wavelengths, but the most intense light is at the yellow wavelengths.
- If graphed, we can see how this looks like.
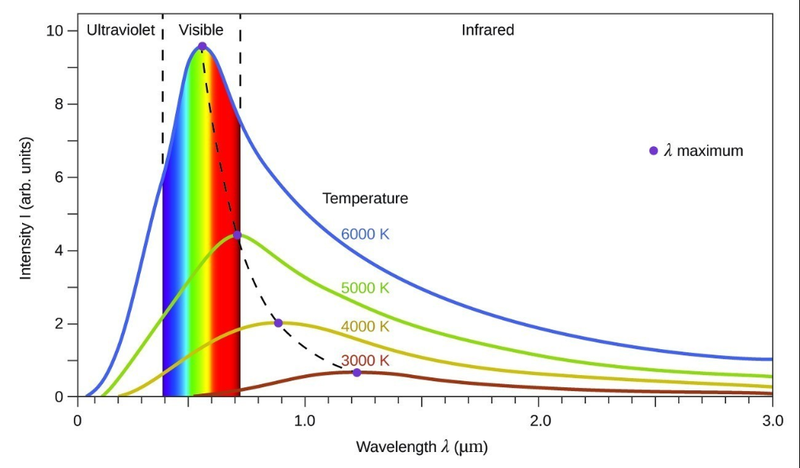
- Maximum intensity occurs at a specific wavelength.
- The graph rises steeply until the maximum point, but drops off slowly after the peak, causing an asymmetrical graph.
- The intensity approaches the x-axis as an asymptote.
- From the graph we can also see that the emitted wavelength is also dependent on temperature.
- Lower temperatures have larger wavelengths for their peaks, but the intensity at the peak is smaller.
- This is why blue fire is hotter than red fire!
Wien's Displacement Law
- Wien's law states that as temperature goes up, the peak wavelength goes down, showing that they have an inversely proportional relationship.
- This also means that:
- peak wavelength * temperature = constant

- The constant is given in the units meters Kelvin.
Sources
https://heloiselenin.blogspot.com/2022/11/19-stefan-boltzmann-law-calculator.html
https://www.collegesidekick.com/study-guides/chemistryformajorsxmaster/electromagnetic-energy-2