What is Thermal Energy?
- Thermal energy is the energy caused by the movement of particles.
- It is directly proportional to temperature.
Heat Transfer
- Temperature (K) is directly proportional to the average random translational kinetic energy of particles in a gas.

- A temperature rise is equivalent to the particles gaining kinetic energy.
Changes of Phase
- A phase change represents a change in particle behavior arising from a change in energy at constant temperature.
- A thermal energy transfer occurs whenever there is a difference in temperature between two bodies.
- Thermal energy is always transferred from an aera of higher temperature to an area of lower temperature, until thermal equilibrium is reached.
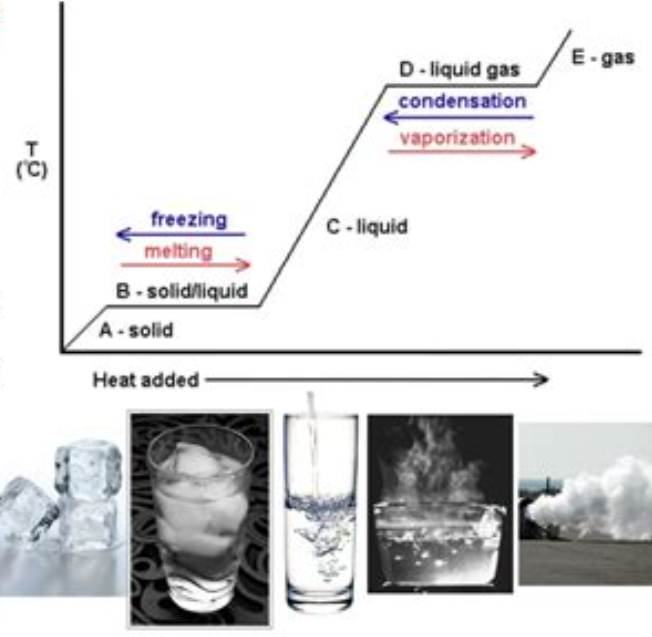
- However, the graph above shows that sometimes transferred thermal energy doesn't cause an increase in temperature.
- Instead, it causes the material to change its phase.
- Once the phase changes, the material continues to heat up as normal.
Internal Energy
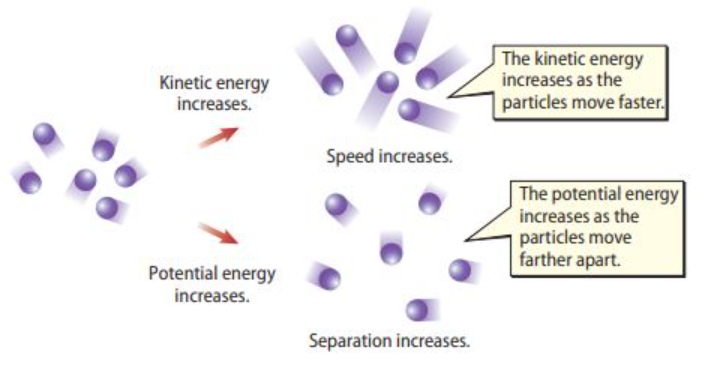
- The internal energy of a substance is the total amount of kinetic energy and potential energy of all of the particles added together.
- Internal energy can be changed in two ways:
- An change in temperature will cause an change in kinetic energy.
- A change in phase will cause an change in potential energy.
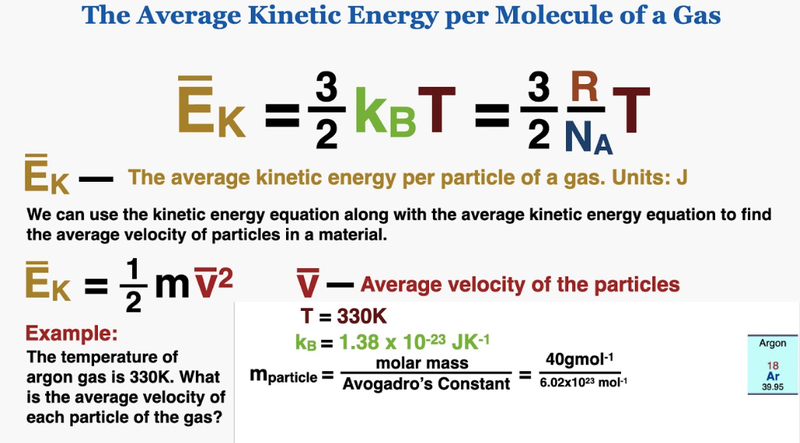
The total kinetic energy of particles can be calculated if we know the amount of moles.
Internal Energy and Pressure
- If we heat up a gas in a container:
- The particles will gain kinetic energy and move faster.
- They will collide with the sides of the container more often, resulting in increased pressure.
- This could cause the container to expand or break.
Specific Latent Heat
- The specific latent heat of a substance is defined as:
- The amount of heat energy needed to change the state of one kilogram of a substance without changing its temperature.
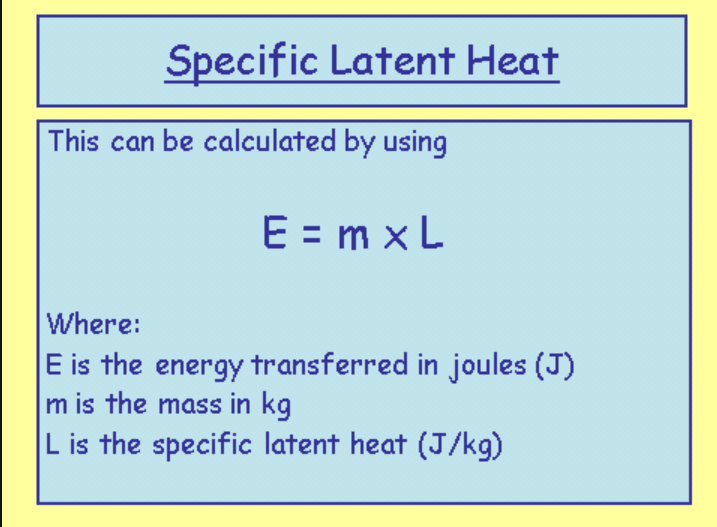
- Keep in mind that Q is often used instead of E to represent thermal energy.
- This formula is applicable in situations that include:
- Freezing or solidifying
- Condensing
- Melting
- Evaporating or boiling
- When calculating the specific heat latent it is often assumed that no energy is lost during transfer.
Heating and Cooling
- When heating up, for example, liquid in a beaker heated with a Bunsen burner, the liquid gains internal energy and gets hotter.
- The three things that the increase in temperature depends on are:
- The amount of liquid
- The mass of the liquid
- The amount of heat energy going in to the liquid
- How hot the Bunsen burner is and how long it is on for
- The substance being heated
- Some substances are easier to heat than others.
- The amount of liquid
- We can see that the rise in temperature depends on the mass, the amount of energy given, and the nature of the substance heated.
- These three factors can be put into an equation:
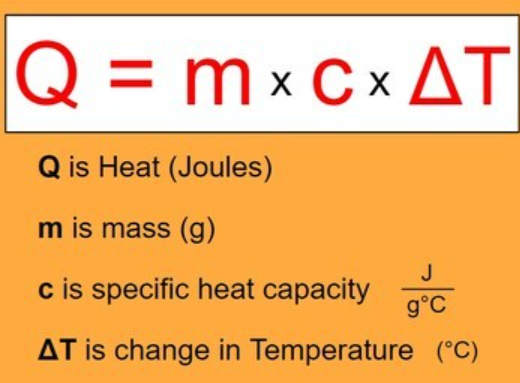
- This equation is rearranged from:
- ΔT = Q/mc
- Thermal energy is proportional to increasing temperature.
- Mass and specific heat capacity are inversely proportional as materials with greater mass and higher specific heat capacity are harder to heat up.
- ΔT = Q/mc
Specific Heat Capacity
- Specific heat capacity can be thought of as the ability of an object to store heat energy.
- It's proper definition is:
- The amount of energy needed to raise the temperature of 1kg of a substance by 1°C.
- It is measured in joules per kilogram Celsius.
Heat Loss
- In an insulated situation, where no heat escapes into the surroundings:
- Thermal energy lost is always equal to thermal energy gained.
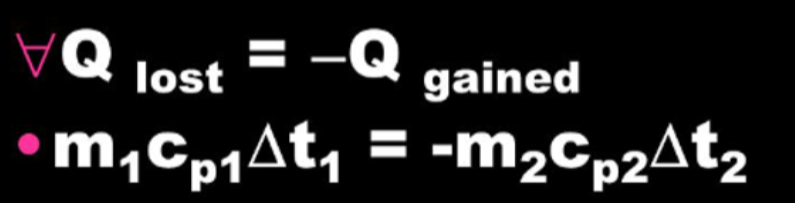
- This law is used when solving calorimetry problems for finding the final temperature of a system.
- For example, consider a hot metal sword dipped into water.
- The thermal energy lost by the sword as it cools is equal to the thermal energy gained by the water as it heats up.
- However, water has a very high specific heat capacity, so it wont increase in temperature as much as the sword loses temperature, despite gaining the same thermal energy.
- For these kinds of questions you need to assume that no energy is lost to or gained from the surroundings.
Sources
https://www.youtube.com/watch?v=pQMHV8u2CLQ
https://punchlistzero.com/is-thermal-energy-potential-or-kinetic/
https://ar.inspiredpencil.com/pictures-2023/heat-capacity-equation