Particle Theory and Wave Theory
- Newton proposed that light travelled as a wave as it exhibits wave behavior, such as reflection, refraction and diffraction.
- Planck argued that the photoelectric effect shows that energy depends on frequency, not amplitude.
- An electron can be released using only one photon as long as it has the right frequency.
- He called this the "particle behavior of waves".
- This means that light and other electromagnetic waves can behave as particles (photons).
- Can particles also behave like waves?
Evidence for the Wave Nature of Matter
- Recall the interference pattern obtained when a wave is passed through a double slit.
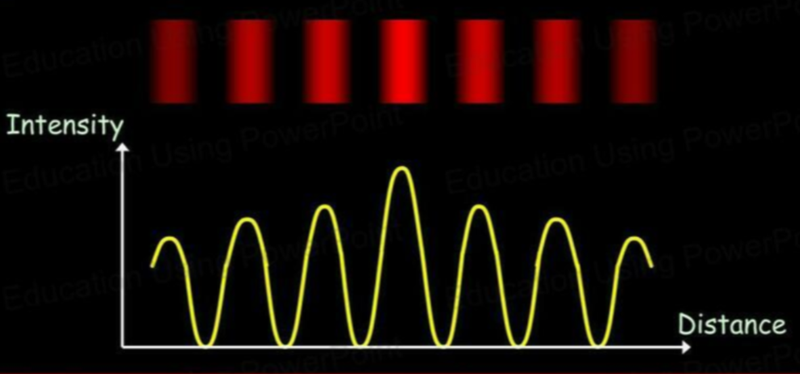
- The highest levels of diffraction are seen when the size of the slit is close to the size of the wavelength.
- John Thompson won the Nobel Prize for discovering the electron in 1897.
- His experiment showed what happened when electrons are aimed at a graphic crystal.

- A diffraction pattern!
- Electron diffraction patterns are seen when electrons are passed through graphite crystal, giving evidence for the wave behavior of matter.
- Diffraction is seen because the distance between atoms is of the same order as the de Broglie wavelength of the electrons.
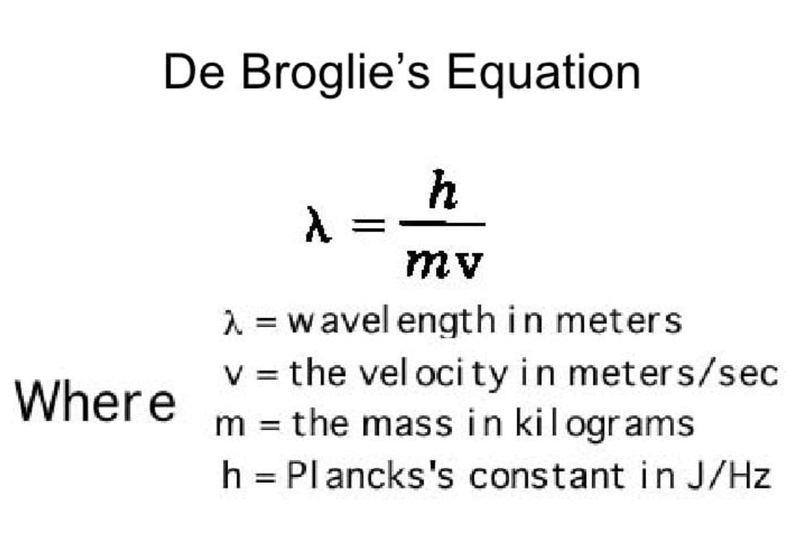
- de Broglie stats that "if light could behave as a particle, then particles must also be able to behave as waves".
- His equation relates the wavelength of an electron to Planck's constant and the momentum of the electron.
- De Broglie's hypothesis was verified in 1927 by physicists Clinton Davisson and Lester Germer.
- They did an experiment that involved firing a beam of electrons at a diffraction grating, which was a single crystal of nickel.
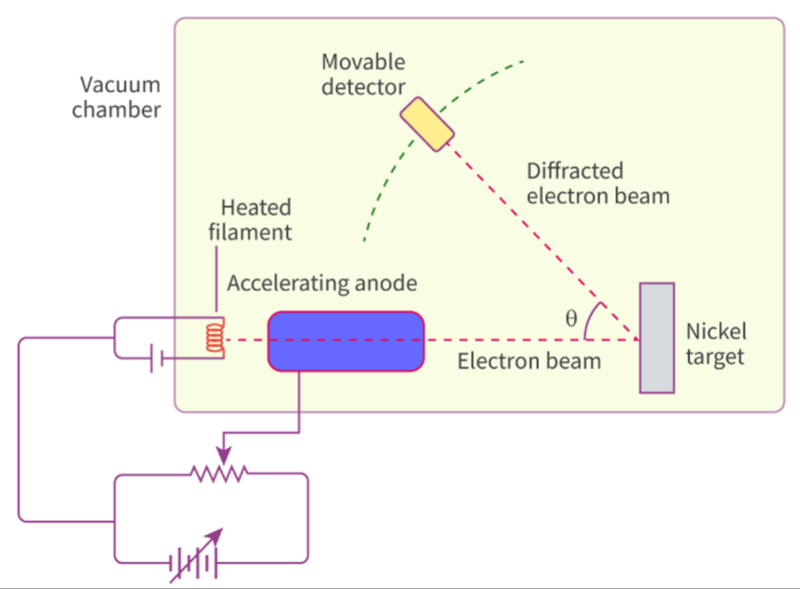
- The beam of electrons was diffracted and a moveable detector measured the intensity of the diffracted beam of electrons at different angles.
- The data was plotted on a graph:
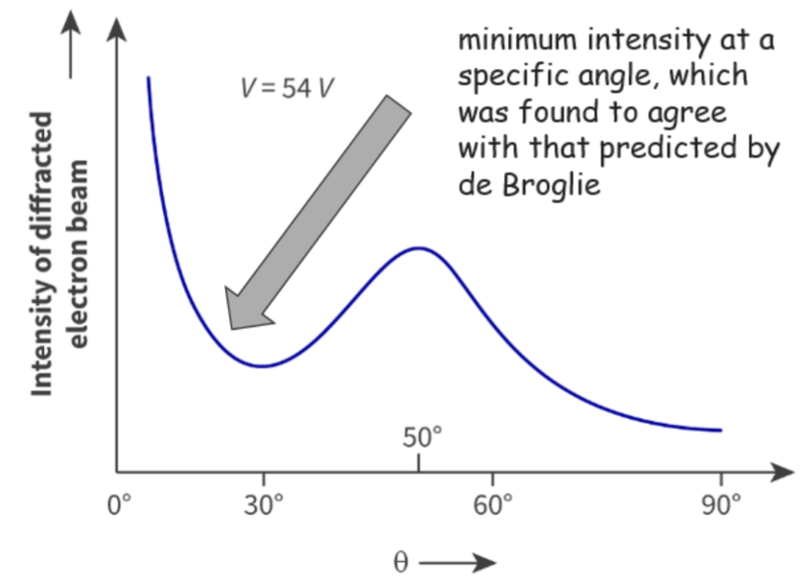
- The effect of diffraction is greatest when the size of the gap is similar to, or greater than, the wavelength.
- The diffraction of electrons, which provides evidence of the wave behavior of matter, is best observed when a thin layer of graphite or single crystal of nickel is used as the diffraction grating.
- This is because the distance between the atoms is similar in size to the rangeof the de Broglie wavelengths of an electron.
- About 10^-10m.
Wave Particle Duality
- In principle, all particles have wave properties and all electromagnetic waves have particle (photon) properties.
- This is widely known as wave-particle duality.
Compton Scattering
- In 1922, Arthur Compton discovered further evidence that supported the theory that light could behave as particles.
- Compton fired X-rays at a carbon target and he observed that the scattered wave had a longer wavelength (and a lower frequency) than the incident wave.
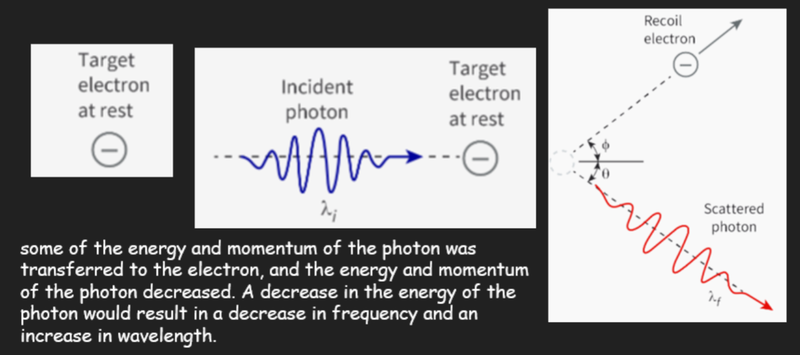
- As the energy of a photon is defined as E = hf, it means that the photon must have lost energy, by transferring it to the electron.
- The essential feature of Compton scattering is that it cannot be explained by considering the incident X-rays to be waves.
- A particle model is needed:
- An individual photon with momentum p = h/λ collides with an individual electron.
- A particle model is needed:
- Applying the laws of conservation of momentum and energy leads to the following equation, which predicts the change in wavelength, Δλ, of the photons:

Sources