The Photoelectric Effect
- The primary evidence for light as a particle is the photoelectric effect.
- This is the emission of photoelectrons from a metal when electromagnetic radiation is incident.
- Consider a gold-leaf electroscope with a charged top.
- A piece of zinc is put on top and UV light is shined at it.
- The UV light causes the zinc to emit electrons.
- This process is called "photoelectric emission", and the emitted electrons are called photoelectrons.
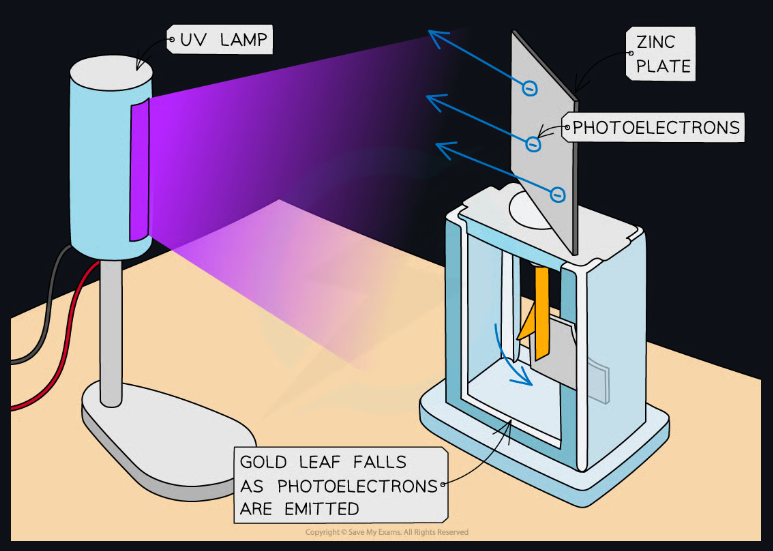
- However, if red light is used instead, no effect occurs.
- Although electrons would eventually be naturally emitted through other processes.
- If brighter red light is used, there is still no effect.
- This shows that the energy of the incoming photon only depends on its frequency.
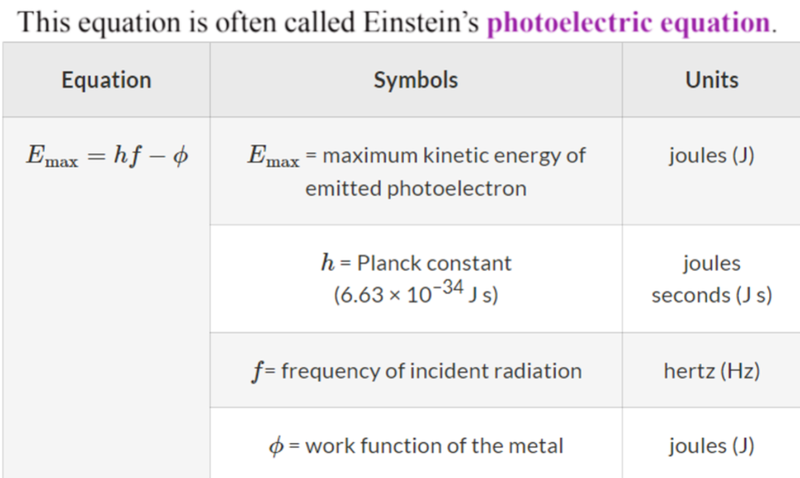
- Thus we can say that:

- This is the same formula used when an atom jumps between energy levels and emits a photon!
Observations from Investigations into the Photoelectric Effect
- If the intensity of the radiation is increased, the charge on the plate increases more quickly because more photoelectrons are being released every second.
- There is no time delay between the radiation reaching the metal surface and the emission of photoelectrons.
- The release of photoelectrons from the surface appears to be instantaneous.
- The photoelectric effect can only occur if the frequency of the radiation is above a certain minimum value.
- The lowest frequency for the emission is called the threshold frequency, f0.
- For a given incident frequency, the photoelectric effect occurs with some metals but not with others.
- This is because different metals have different threshold frequencies.
- The photoelectrons emitted from a particular metal by monochromatic radiation of a known frequency have a range of kinetic energies, up to a well-defined maximum.
Quanta
- For zinc, the photoelectric effect is only seen when UV light, or a light of higher frequency is used.
- Thus the threshold frequency for zinc is f0 = 1*10^15Hz.
- Max Planck proposed that electromagnetic radiation like light, comes in small packets.
- The general name for these packets is "quanta", with the singular "quantum".
- Hence, quantum physics!
- The general name for these packets is "quanta", with the singular "quantum".
- In the specific case of electromagnetic radiation, a quanta is called a "photon" and its energy depends on its frequency, not how bright it is.
- The amount of energy needed to release an electron from a metal is called the "work function" and is given the symbol phi, ϕ.
- Generally, work functions are lower for more reactive metals.
Explaining the Photoelectric Effect: The Einstein Model
- The Einstein model explains the photoelectric effect using the concept of photons.
- When a photon in the incident radiation interacts with an electron in the metal surface, it transfers all of its energy into that electron.
- It should be stressed that a single photon interacts with a single electron and that this transfer of energy is instantaneous.
- Einstein realized that some of the energy carried by the photon was used to overcome attractive forces that normally keep an electron within the metal surface.
- The remaining energy is transferred to the kinetic energy of the newly released photoelectron.

- It can also be used to find the maximum kinetic energy of released photoelectrons.
Experiments to Test the Einstein Model
- The kinetic energy of the photoelectrons can be transferred to electrical potential energy if they are repelled by a negative voltage.
- This experiment was first performed by the American physicist Robert Millikan who investigated stopping voltages.
- The (variable) source of potential difference was connected the "wrong way around" in the which means that there is a negative voltage anode that will repel the photoelectrons, slowing them down.
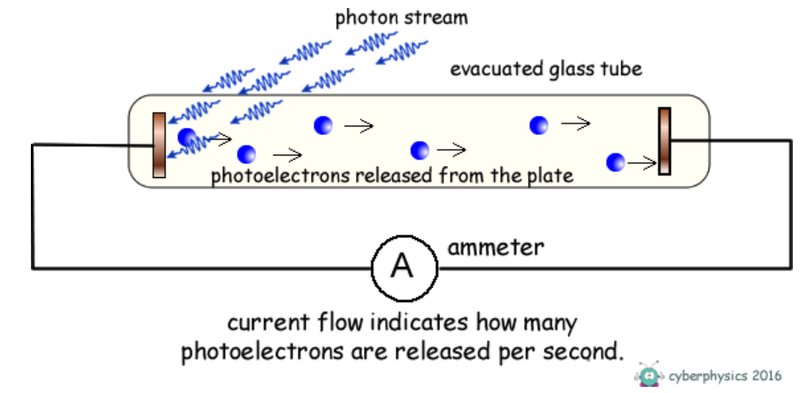
- A battery is often placed in the circuit in the opposite direction to oppose the flow of photoelectrons.

The "Hill" Analogy
- To help us understand this further, let's say the electron is like a ball rolling up a hill.
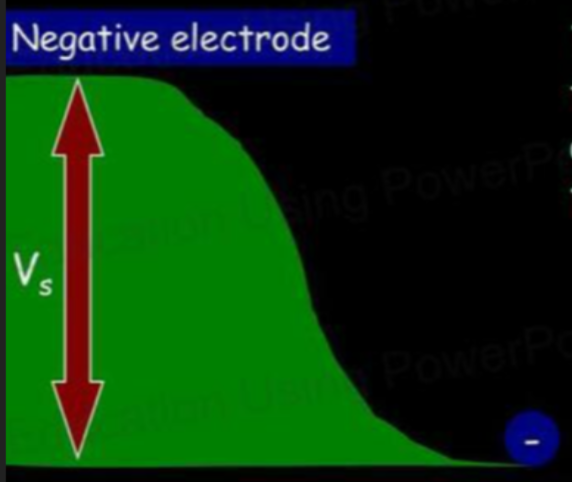
- The amount of potential energy the electron gains is equal to the amount of kinetic energy it had at the start.
- In electric terms, the voltage the electron can work against depends on how much energy it had.

Photocurrent vs Voltage
- If this voltage is large and negative, no electrons will be able to move "up the hill" so the current is zero.
- However, if the voltage is positive, electrons will be "helped up the hill".
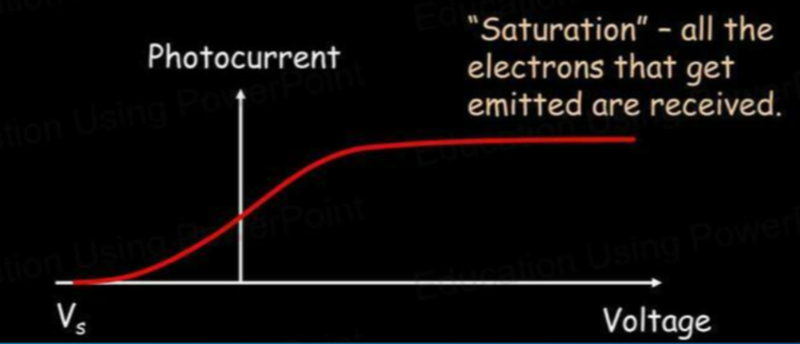
Photocurrents for Different Light
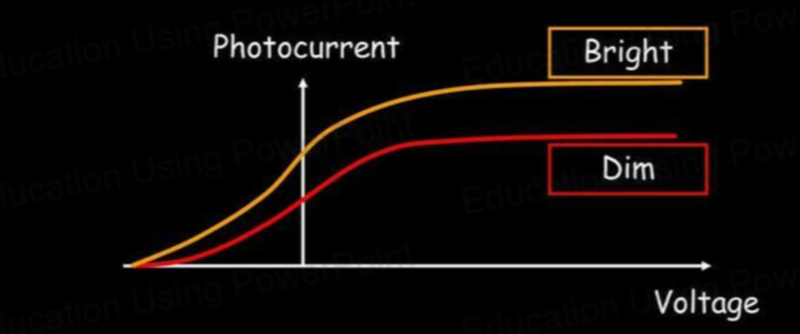
- Different intensities mean that the stopping voltage is the same as the frequency stays the same, but the photocurrent is greater for greater intensities as more photoelectrons are emitted per second.
- For different frequencies the stopping voltage is different.
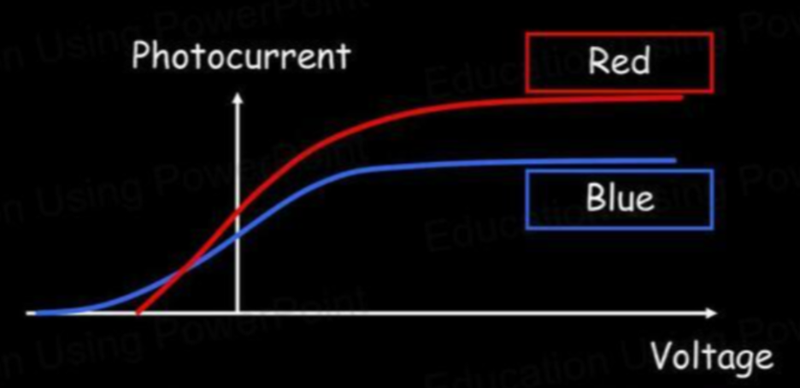
- The stopping voltage is different as electrons emitted by blue light will have more energy (as blue light is higher in frequency).
- Notice that the intensities are the same.
- The different saturations are because more electrons are emitted by red, but with less energy.
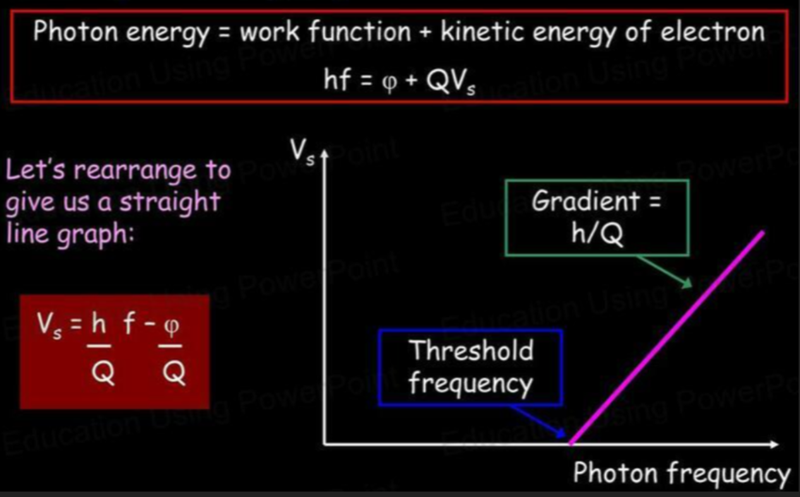
Photon Energy
- By combining the theory of the kinetic energy of an electron and the work function we get:
Sources