Remodeling the Atom
- Over a century ago people thought the atom looked like a sphere of positive charge with negatively charged electrons spread through it.
- It can be thought of as "plum pudding", with the electrons being the bits of plum spread throughout.
- Ernst Rutherford, Geiger and Mardsen conducted and experiment that disproved this idea.
- It is called the "scattering experiment".
Rutherford Scattering Experiment
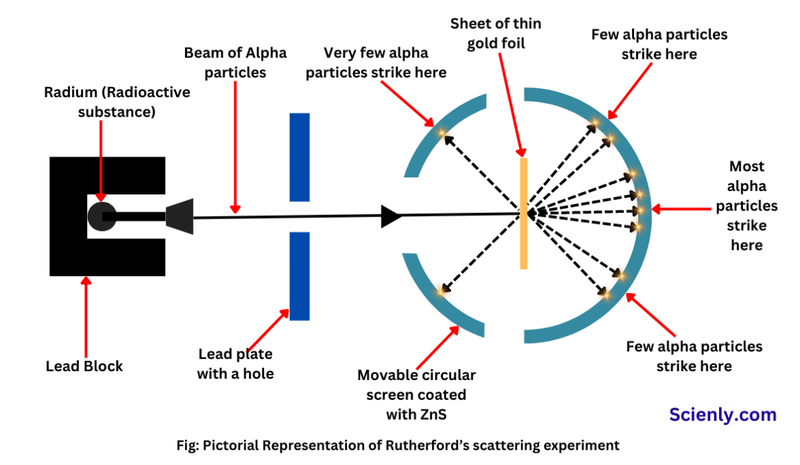
- Alpha particles, with positive charge are fired at thin gold foil.
- The gold foil is as thin as possible (ideally 1 atom thick).
- Most particles passed through the gold foil and landed on the detector.
- However, 1/8000 were deflected by more than 90°.
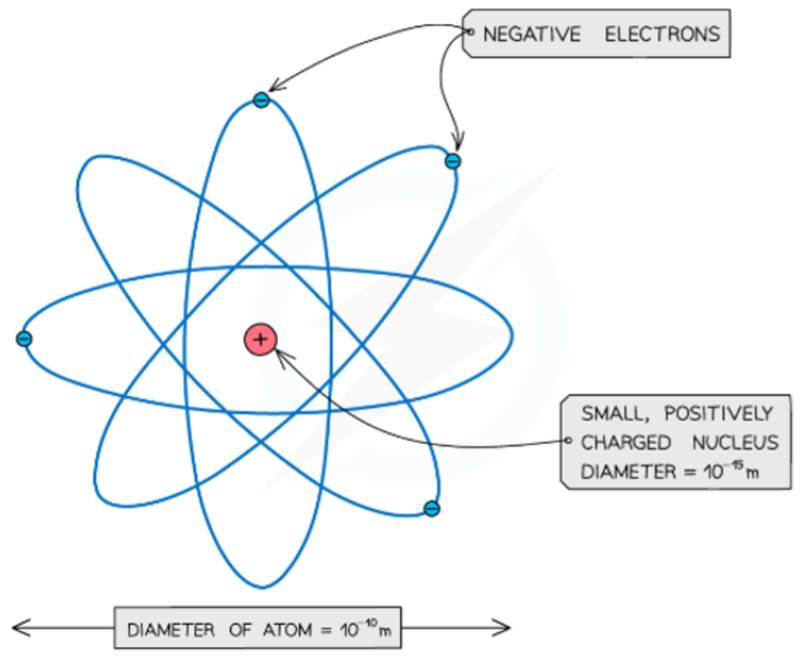
- From this experiment, Rutherford concluded that the atom is made up of a small, positively charged nucleus surrounded by electrons orbiting in a "cloud".
- The center of an atom (the nucleus) must be positively charged in order to repel the positive charged alpha particles.
- Alpha particles that pass close to a nucleus experience a strong electrostatic repulsive force, causing them to change direction.
- Rutherford proposed that the positive charge of the atom was located in the center, and he coined the term nucleus.
Geiger-Mardsen-Rutherford Experiment
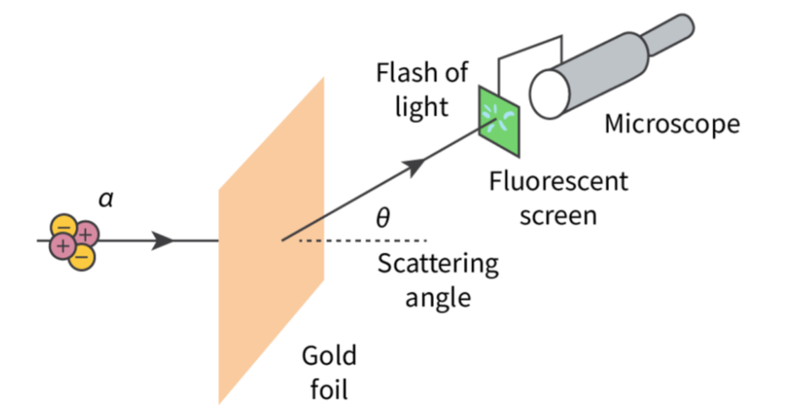
- Alpha particles were fired at a sheet of gold foil and their scattering angle was observed when they landed on a fluorescent screen, resulting in a flash of light.
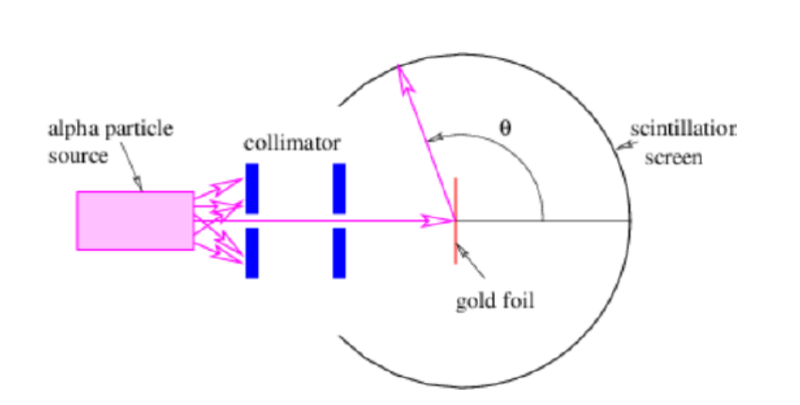
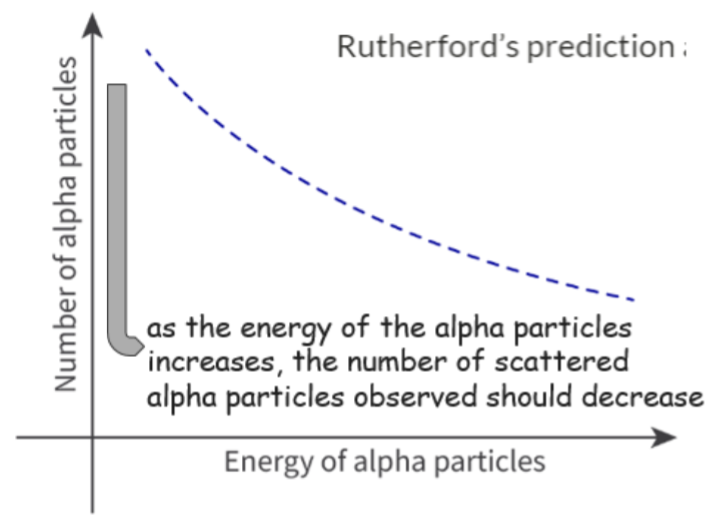
- The experiment showed that the greater the scattering angle, the fewer the scattered alpha particles that were observed.
- The number of scattered alpha particles observed in a particular time interval for a certain angle depends on the energy of the alpha particles.
Distance of Closest Approach
- If an alpha particle is fired at the nucleus of an atom with proton number Z:
- The alpha particle has kinetic energy Ek.
- The alpha particle and the nucleus are far enough apart that there is no electric force between them at the start.
- As the alpha particles approaches, it is repelled by the electric force and decelerates.
- At a certain point the alpha particle will momentarily stop.
- It will have reached its minimum distance from the nucleus.
- We call this distance the distance of closest approach.
- All of the kinetic energy of the alpha particle was transferred to the electric potential energy of the system (nucleus and alpha particle).
- It will have reached its minimum distance from the nucleus.

Deviations from Rutherford Scattering
- Rutherford predicted that the alpha particles and nucleus will repel each other as they are both positively charged.
- Since the nucleus is much heavier than the alpha particle, it experiences negligible deflection, and only the alpha particle changes direction.
- If the alpha particle travels faster and therefore has more energy, the change in the direction will be less, so fewer alpha particles will be detected at a certain angle.
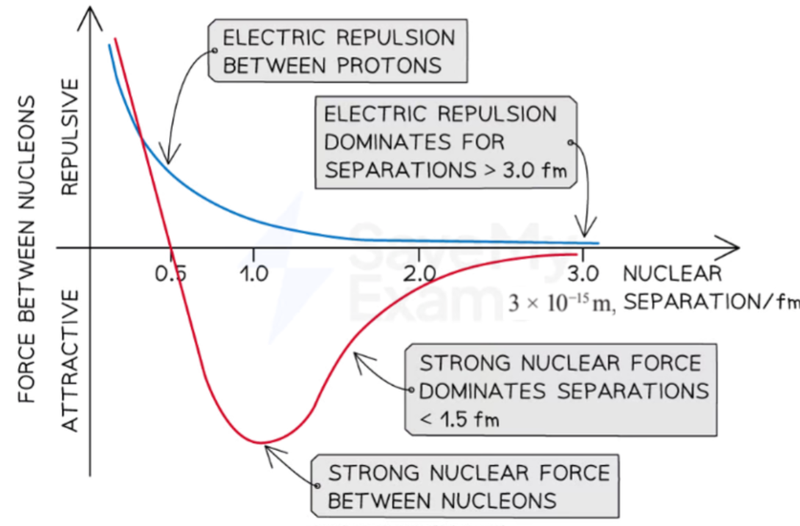
- There is another field force acting between individual nucleons when they are close together:
- This is the strong nuclear force.
- This attractive force overcomes the repulsive forces between positively charged protons, causing alpha particles to scatter far less if they get close enough.
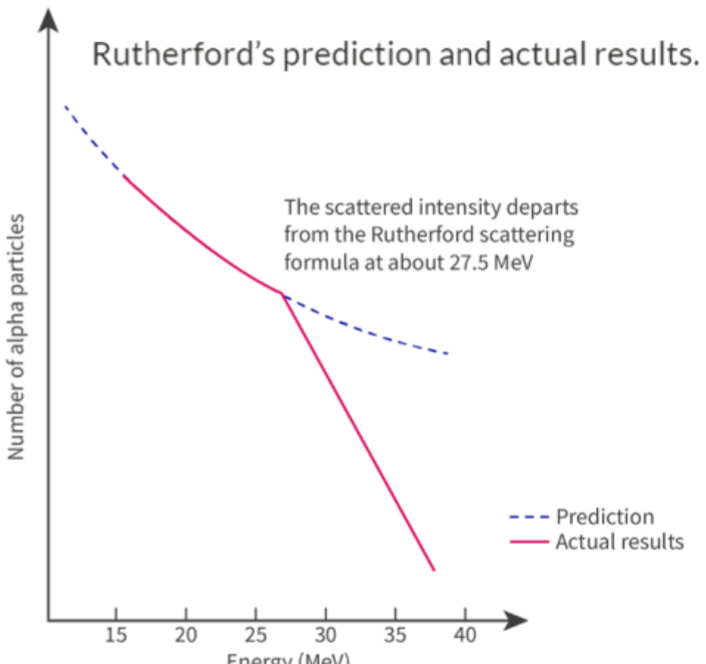
- A very energetic alpha particle can get close enough to the nucleons and it is affected by the attractive strong nuclear force, as well as the repulsive electric force.
The Bohr Model of Hydrogen
- Neils Bohr suggested that electrons can only orbit at specific radii and that these radii are defined in terms of the angular momentum of the electron.

- Where:
- mvr = the angular momentum of the electron, L
- n = a whole number, called the "quantum number"
- h = Planck's constant
- An electron's angular momentum is quantized, meaning it can only have discrete values.
- The angular momentum is quantized, as it is always some value of n * constant.
Relating the Bohr Model to Spectra
- Bohr's model can also be applied to energy levels.

- This equation predicts that the energy levels will become closer together as the energy increases, which we can see on the diagram for spectra of an atom.
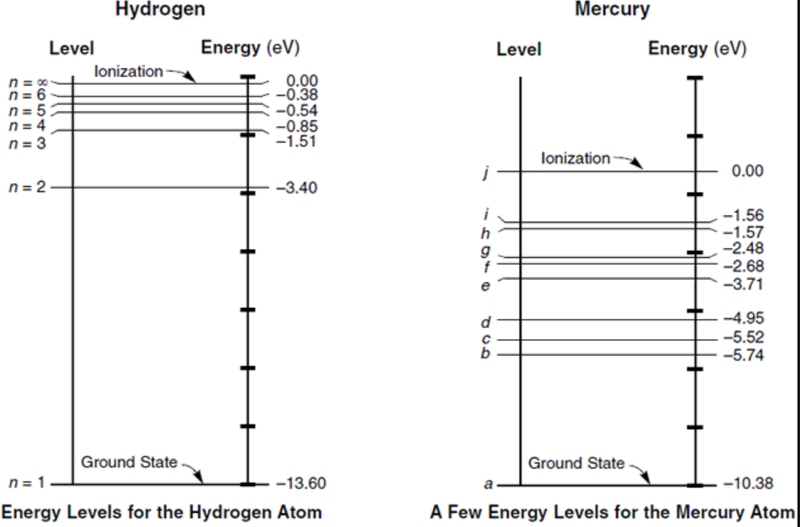
- Bohr introduced this equation for the energy levels because:
- Electrons, as they are travelling in circular motion, are always accelerating.
- Therefore they must radiate energy.
- If they lose energy, they would spiral into the nucleus.
- But electrons have discrete energy levels.
- The only orbits where electrons do not radiate are those that satisfy Bohr's equation.
- Electrons, as they are travelling in circular motion, are always accelerating.
Sources