What is Radioactivity?
- Radioactivity is spontaneous changes within an unstable nucleus that result in the emission of a particle or a high-energy photon.
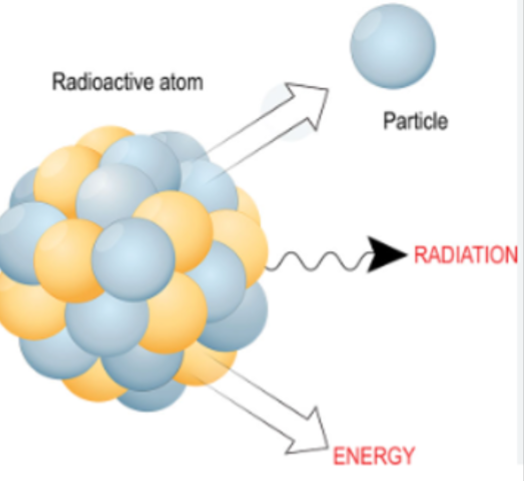
- Transmutation is when a nuclide changes to form a different element after emitting a particle.
- A daughter product is the resulting nuclide after a radionuclide, the "parent", emits a particle.
- Radioactive describes a substance which contains unstable nuclei which will emit radiation.
- Isotopes are atoms with a different number of neutrons.
- Isotopes tend to be radioactive, with the isotopes with the greatest amount of neutrons being very radioactive and having short half lives.
Radioactive Decay
- Uranium has several isotopes/nuclides which are all unstable, and thus radioactive.
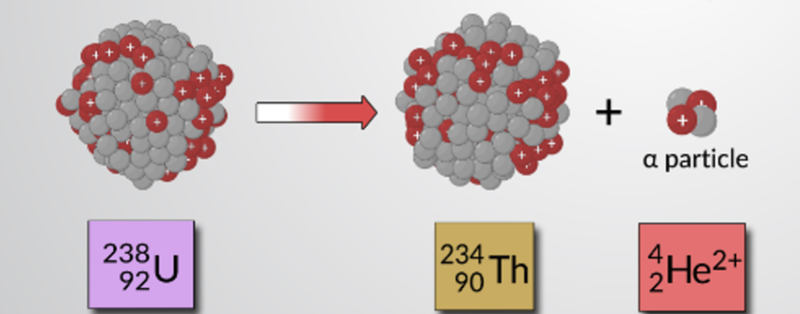
- The above image shows how uranium decays into thorium and an alpha particle.
Radioactivity Experiments
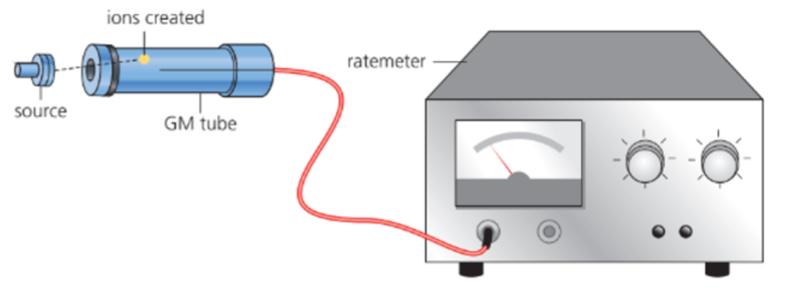
Geiger-Muller Tube
- The Geiger-Muller tube is an apparatus used with a counter or ratemeter to measure the radiation from a radioactive source.
Count Rate
- Count rate, or radioactivity, is the number of nuclear radiation events detected in a given time
- Events per minute or per second.
- A ratemeter is a meter which is connected to a Geiger Muller tube or similar device to measure the rate at which radiation is detected.
Source
- A tiny amount of radioactive nuclide is contained in the source.
- When nuclear radiation emitted by the source enters the Geiger-Muller tube through the end "window", it causes ionization of the gas inside and a sudden tiny burst of current.
- These events are counted by an electronic counter or ratemeter, and the results are expressed as a radioactive count.
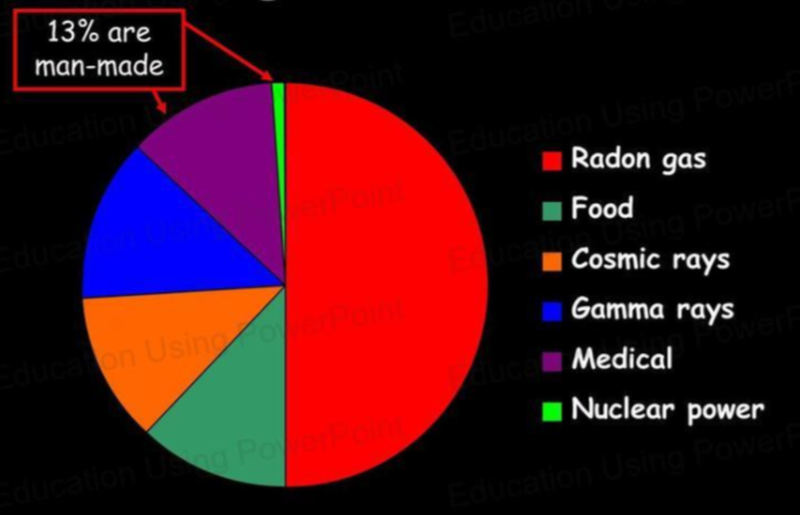
Background Radiation
- While we think of radiation as only caused by nuclear bombs and power plants, it is actually a very common phenomenon at low levels.