Nuclear Stability
- In a stable nucleus, we can consider that the very short-range attractive strong nuclear forces are balanced by the longer range repulsive electric forces.
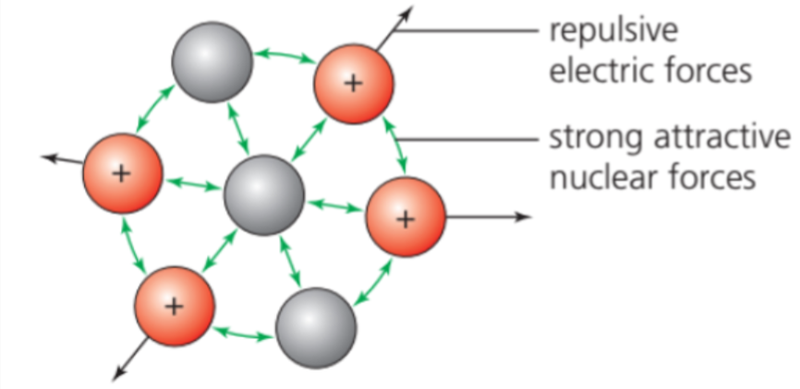
- A nucleus will be unstable if it has:
- Too many neutrons
- Too many protons
- Too many nucleons (too heavy)
- Too much energy
- The stability of a nuclide depends on the ratio of neutrons to protons (N/Z) in its nucleus.
- An unstable atom wants to become stable.
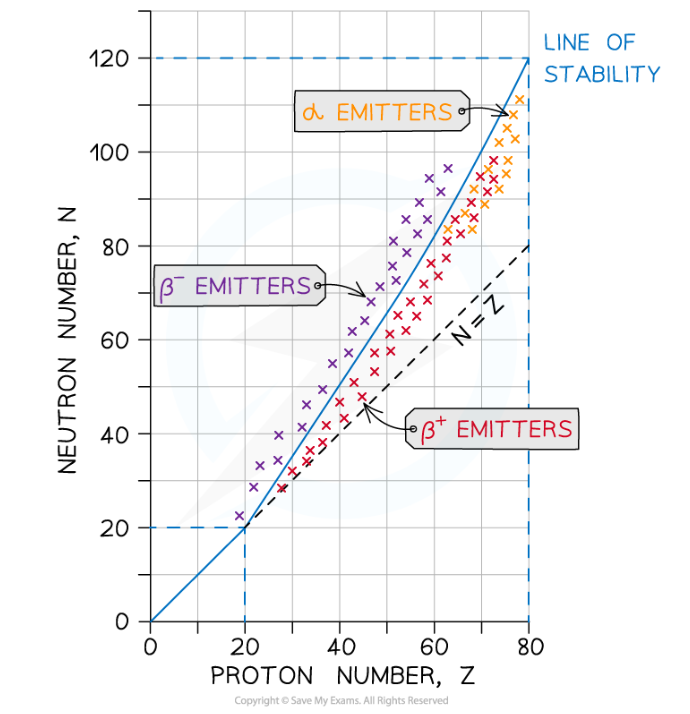
- Lighter elements with, fewer protons and neutrons, tend to be much more stable than heavier elements with many protons and neutrons.
- For nuclides with Z<20, stable nuclides have N/Z ≈ 1.
- This means they tend to have around the same number of protons has neutrons.
- For larger nuclides, N/Z gradually increases to about N/Z ≈ 1.5.
- This means they have more up to 1.5 times more neutrons than protons.
- Adding neutrons to larger nuclei with A > about 60 increases the total binding energy, but has little effect on the binding energy per nucleon.
Nuclear Energies
Alpha Particle Spectrum
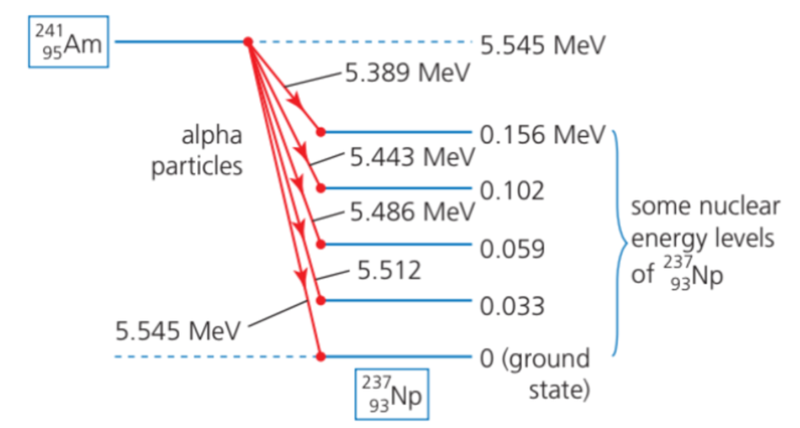
- Many radionuclides which emit alpha particles, emit them with only one precise energy.
- However, some radionuclides can emit alpha particles with different energies as displayed in an alpha particle spectrum.
- The spectrum of nuclear radiations emitted from an unstable nucleus describes the relative numbers of particles emitted with different energies.

- The energy of any emitted alpha particle can only have one of these energies.
- Alpha particles with different energies are possible because the nucleus of neptunium-237 can be left in its ground state, or in one of several discrete excited states.
- The energy that isn't left in the neptunium is carried by the alpha particle.
Gamma Ray Spectrum
- Consider the previous example: after the alpha decays, four excited states of neptunium-237 nuclide can be seen.
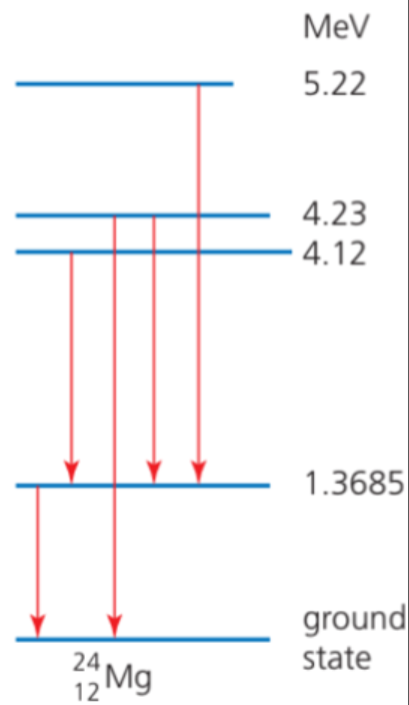
- When a nucleus changes from an excited state to a lower energy level, a gamma ray photon will be emitted.
- Nuclear energy levels are similar to electron levels.
- The nucleus cannot emit a continuous range of gamma raysl.
- The discrete energies of the photons again provide evidence for discrete energy levels within nuclei.
- Nuclear energy levels are similar to electron levels.
- The discrete energy levels of nuclei are the reason why alpha particles and gamma rays are emitted with discrete energies.
Beta Particle Spectrum
- The spectrum of beta particles emitted from a particular radionuclide is very different from alpha particles.
- It is a continuous spectrum, unlike alpha particles, which have discrete energies.
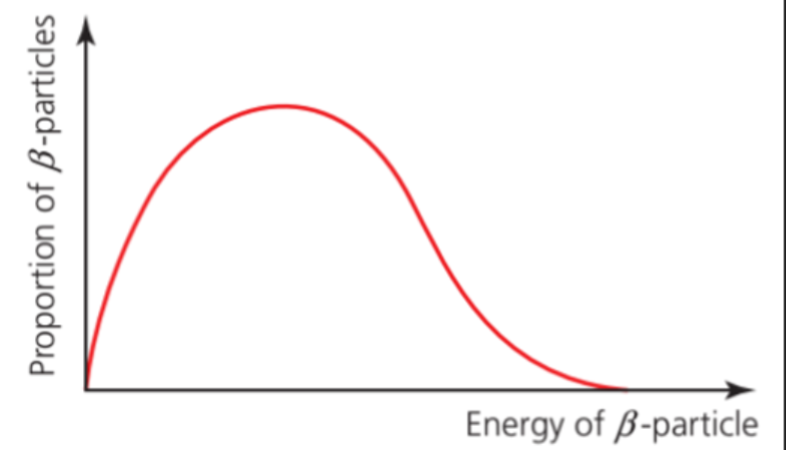
- The emission of a beta particle involves another particle (an undetected neutrino or antineutrino).
- These particles may travel in random directions, so that a continuous range of beta particle energies is possible.
- The existence of the neutrino was proposed in 1930 by the physicist Wolfgang Pauli and it was confirmed in 1956.
- Neutrinos are incredibly small elementary particles and are neutral so they don't interact with other particles.
- Many neutrinos can travel in straight lines for millions or even billions of years.
Evidence for the Neutrino
- The anti-neutrino is the antiparticle of a neutrino.
- Electron anti-neutrinos are produced during beta-minus (electron) decay.
- Electron neutrinos are produced during beta-plus decay.
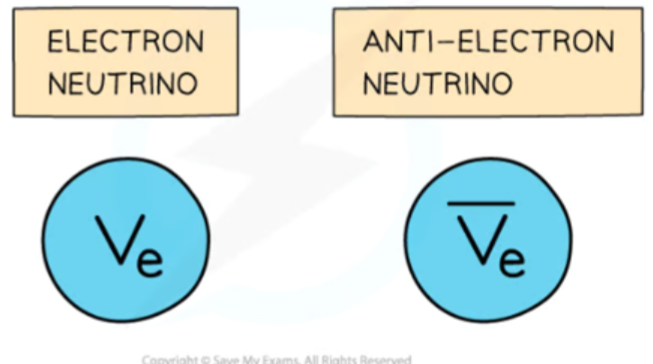
- The energy released in beta decay is shared between the beta particles (electrons or positrons) and neutrinos (or anti-neutrinos).
- The principle of conservation of momentum and energy applies in both alpha and beta emissions.
Sources