What is Radioactive Decay?
- Radioactivity is a random and spontaneous process.
- This means that radioactivity cannot be controlled and occurs without a cause.
- For a sample of a radioactive isotope that decays into a stable isotope, the number of decays per unit time (activity) decreases over time.
- The activity, A, of a radioactive source is the total number of nuclei decaying every second.
- The unit for activity is the becquerel, Bq, where 1 Bq equals 1 decay per second.
Half Life
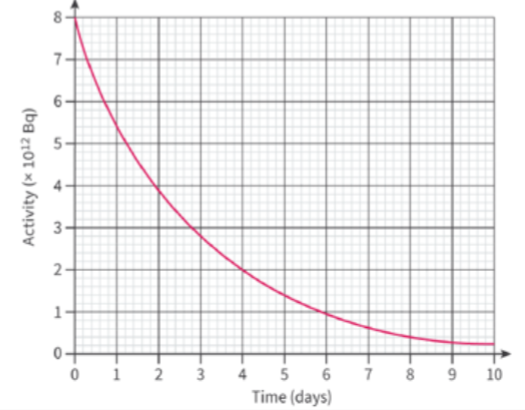
- The decay of radioisotopes can be used to measure the material's age.
- The half-life of an atom is the time taken for half of the radioisotopes in a sample to decay.
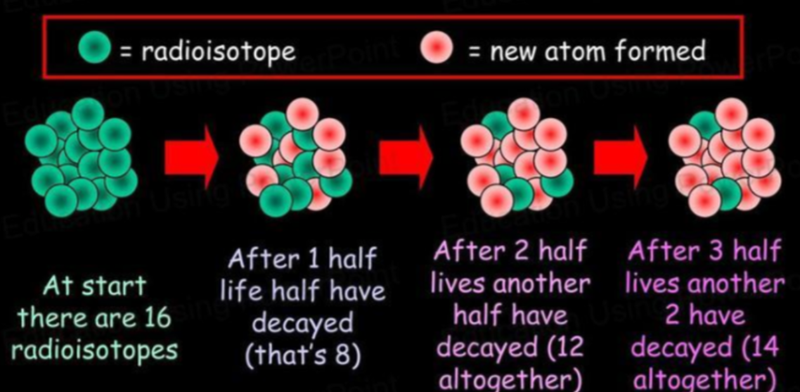
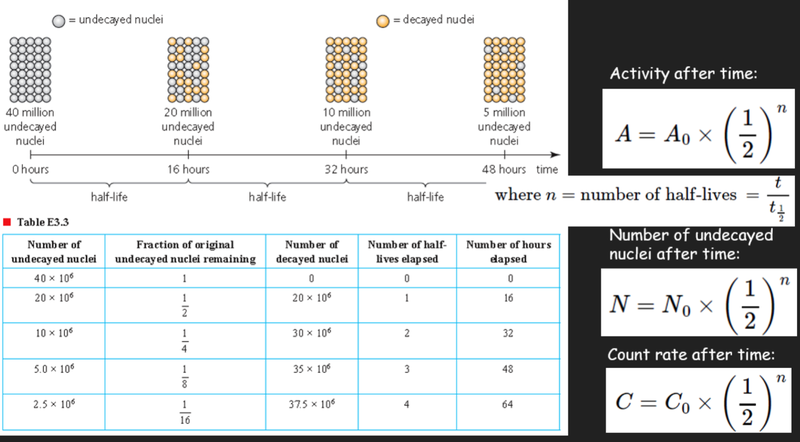
- Because the amount of original radioisotopes decreases by half every time, there is a consistent pattern we can use for formulae:
Experimental Determination of Half-Life
- In principle, the half-life of any radionuclide can be determined from a graph of the count-rate, activity or number of undecayed nuclei against time.
- Although it is often easier to work with count rate or activity.
Practical Uses of Radionuclides
- Radioactive substances have a wide range of uses including:
- Diagnosis of illness
- Cancer treatment
- Food preservation
- Sterilization of medical equipment
- Determining the age of rocks
- Locating faults in metal structures, such as pipes
- Carbon dating (using radioactive carbon isotopes)
- Determining thicknesses
- Smoke detectors
Radioactive Decay Law
- The number of radioisotopes that will decay clearly depends on the number of radioisotopes present at that point in time:

- Thus activity, or the number of decays per unit time, can be calculated using:
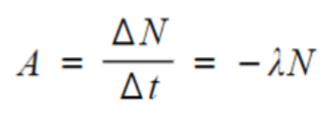
- The greater the decay constant, the greater the activity of the sample.
- The activity depends on the number of undecayed nuclei remaining in the sample.
- The minus sign indicates that the number of nuclei remaining decreases with time.
- Several additional formulae can be used to show the relationship of activity, the number of remaining nuclei and count rate.
- These all use the constant e, or Euler's number.
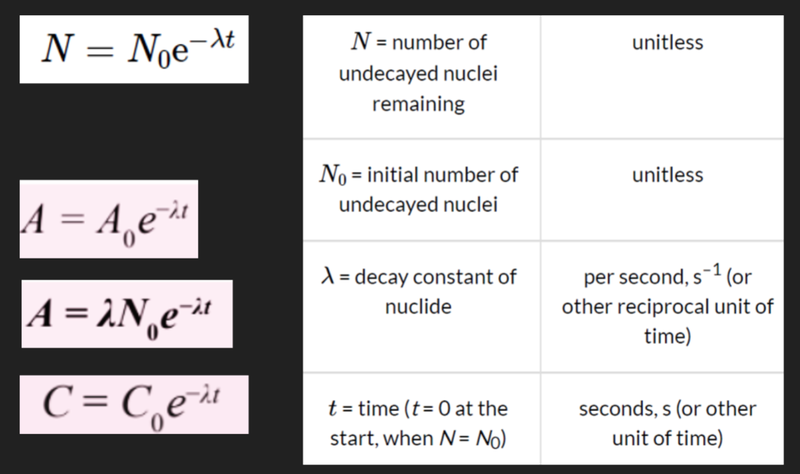
Decay Constant and Half-Life
- We can relate the formula for half life and the formula for the decay constant to find their relationship.
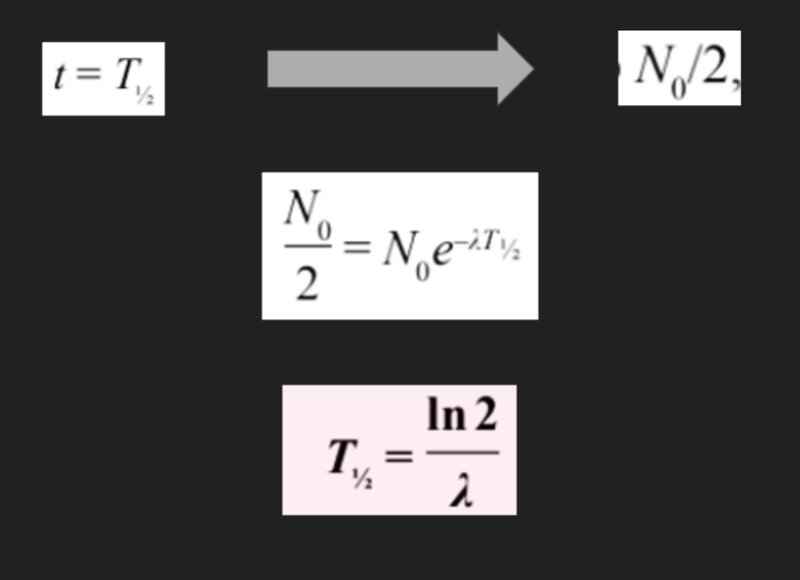
Experimental Determination of Half Life
- For radioisotopes with relatively short half lives, we can create graphs using the radioactive decay laws by rearranging the formulas.
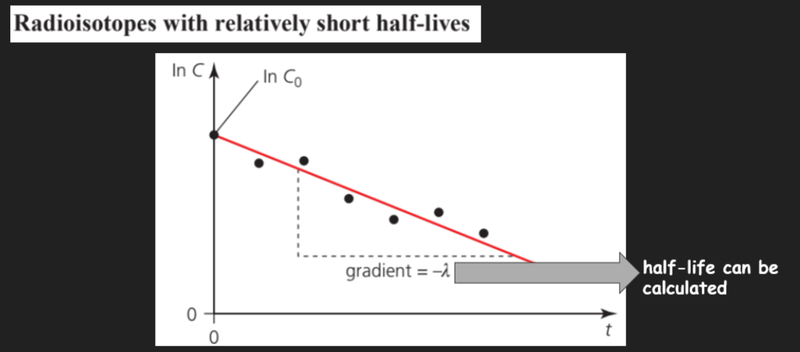
- The gradient of this graph should be the decay constant, which we can use to find the half-life of a substance.
- For radioisotopes with relatively long half-lives the count rate does not change during the course of an experiment, as some can take billions of years to decay!
- Therefore we cannot use the count-rate equation.
- Instead:
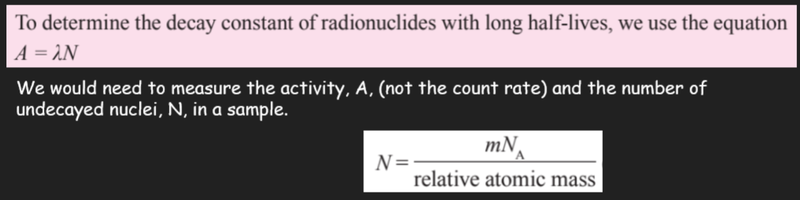
Sources
https://en.wikipedia.org/wiki/List_of_radioactive_nuclides_by_half-life#1030_seconds_(quettaseconds)