The Mass of an Atom
- We know that an atom consists of a positively charged nucleus surrounded by orbiting electrons.
- Electrons have negative charge and nearly zero mass.
- Protons have positive charge and the same mass as a neutron.
- Neutrons have neutral charge and have the same mass as a proton.
- Atoms always have the same number of protons and electrons so they are neutrally charged.
The Charge of an Atom
- In the Rutherford model of the atom, the electrons orbit the nucleus because of the centripetal force provided by the electrical attraction between opposite charges.
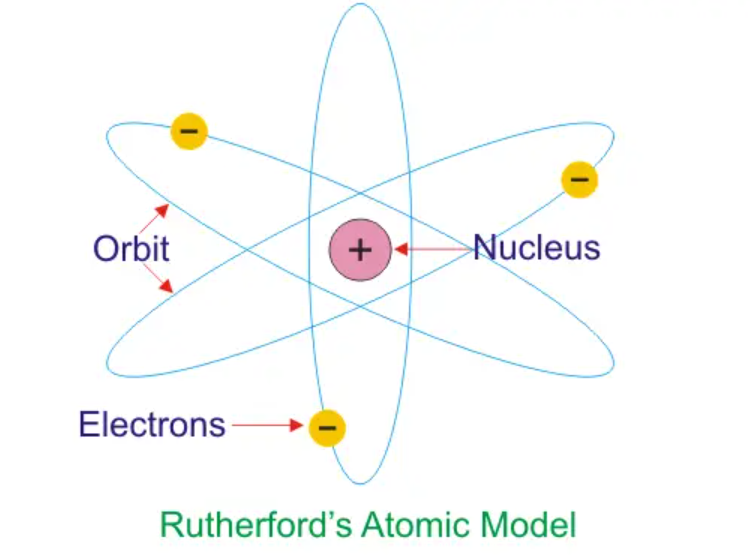
- If the electrons were not in circular motion, the electrostatic force of attraction would accelerate them towards the nucleus.

Composition of the Nucleus
- The term nucleon is used to describe a particle in the nucleus of an atom which is either a proton or a neutron.
- After it was proposed that the nucleus was composed of separate particles (protons and neutrons), there was an obvious question to ask:
- What forces are there between these particles that holds them so closely together?
- In particular it was known that there is a very large repulsive force between protons, as the following approximation demonstrates:
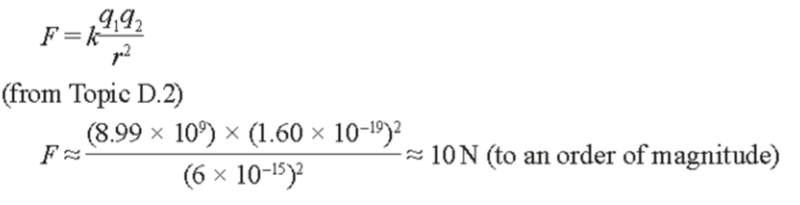
- Which is an incredibly large force on the atomic scale.
- Keep in mind atoms have very little mass, and as F=ma, 10N should send these protons flying apart!
- To oppose this repulsive force, we now know that there is a very short-range strong nuclear force (attractive) between the nuclear particles.
- This force is a lot stronger than the repulse force of protons, as it holds the nucleons together.
Nuclear Radius
- The radius of the nucleus depends on the nucleon number A of the atom.
- The greater the number of nucleons a nucleus has, the greater the space the nucleus occupies, hence giving it a larger radius.
- The exact relationship between the radius and the nucleon number can be determined from experimental data, such as Rutherford scattering.
- By doing this, physicists were able to deduce the following relationship:

Nuclear Density
- The densities of all nuclei are approximately the same.
- This is because as nucleons have constant mass and volume and stick very close together in the nucleus.

- The remaining quantities in the equation are all constants.
- This shows the density of the nucleus is:
- The same for all nuclei.
- Independent of the radius.
- Nuclear density is significantly larger than atomic density which suggests:
- The majority of the atom's mass is contained in the nucleus.
- The nucleus is very small compared to the atom.
- Atoms must be predominantly empty space.
Radii of Electron Orbits
- As the following mathematics shows, the Bohr model combined the classical physics of circular motion and the force of electric attraction with quantum concepts in order to predict the radii of orbits and the energy levels in hydrogen atoms.
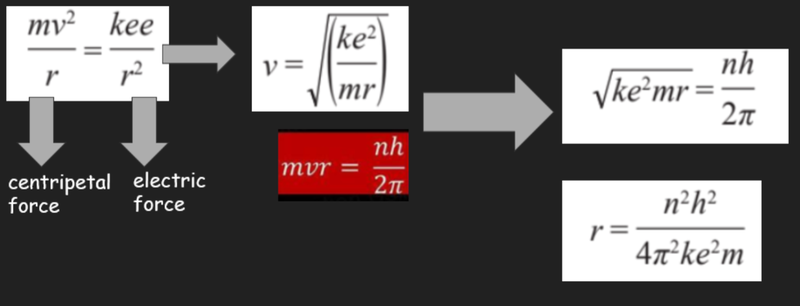
Total Energy of the Atom
- The total energy of an atom can be found by summing the kinetic energy of the electrons, the kinetic energy of the protons and the electric potential energy (energy level of the atom).
- As the protons are stationary, they have no kinetic energy.
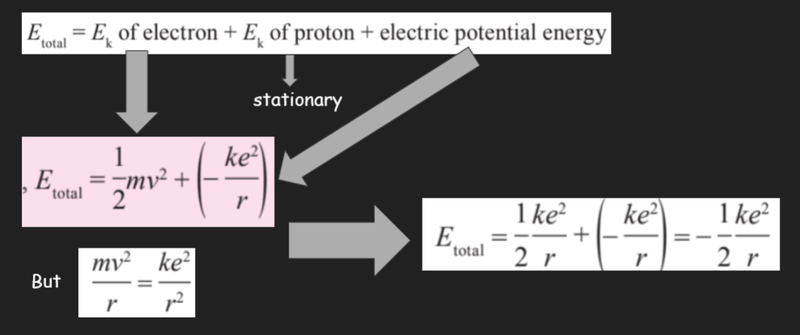
Sources