What is the Particle Theory?
- The particle theory aims to explain the properties of solids, liquids and gases by looking at how the particles they are made of behave.
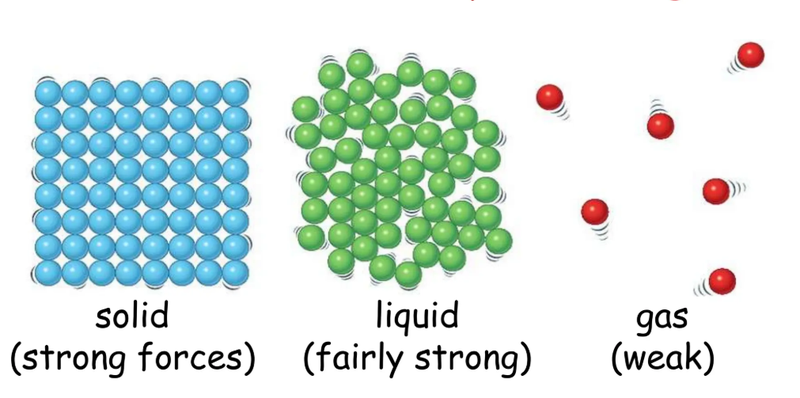
Solids
- In a solid the particles vibrate around a fixed position.
- There is a strong force of attraction between each particle and they are every close together.
Liquids
- In a liquid the particles are close together but can move in any direction.
- They won't keep a fixed shape likes solids do and can change shape to fit in a container.
Gases
- In a gas the particles are very far apart and move quickly in all directions.
- They often collide with each other and because they are far apart they can be easily compressed.
Kinetic Theory of Matter
- The kinetic theory of matter states that:
- All matter is composed of a very large number of small particles that are in constant motion.
- Their motion depends on their temperature, which is the energy within particles.
- Increases in temperature increase kinetic energy of particles.
- Changes in phase (solid->liquid->gas) increase the potential energy of particles.
Density
- Density is defined as unit mass per unit volume.
- As mass can be used to define the amount of substance, and volume the area it takes up, density is the measure of how tightly packed a substance is.
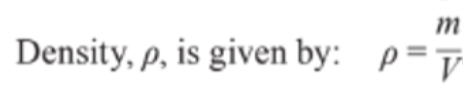
Sources
https://www.slideshare.net/slideshow/particle-theory-notes-and-activities-for-grade-7/270160625