What is Nuclear Notation?
- There are a lot of different elements, so it is important that there is an easy way to differentiate them and quickly see their mass and atomic numbers.
- For this we use the nuclear notation.
- A periodic table uses this notation!
- Elementary particles are particles that have no internal structrure.
- They are not composed of other particles.
- Elementary particles include neutrons, protons and electrons that make up an atom.
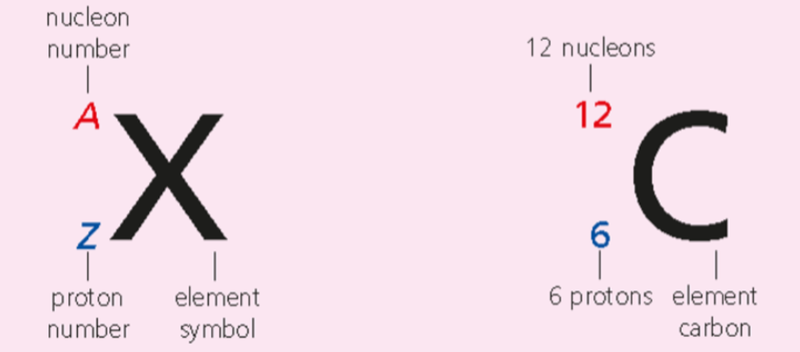
- Nuclear notation is used to indicate elementary particles.
- The proton number, Z, is the number of protons in the nucleus.
- As a particle has the same amount of electrons as protons, this also serves as our electron number.
- The nucleon number A, is the total number of protons and neutrons in the nucleus.
- The neutron number, N, is the number of neutrons in a nucleus.
- The neutron number isn't generally included in the nuclear notation, so it can be found through the simple formula: N = A - Z.
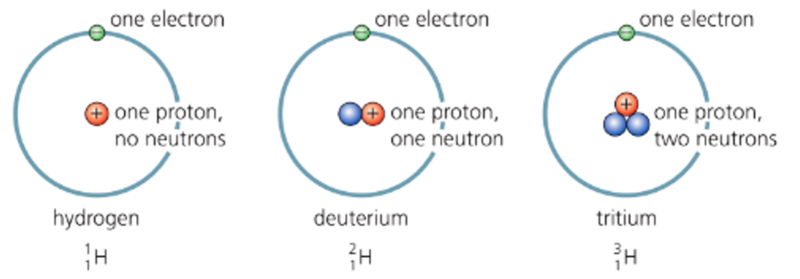
Isotopes
- An isotope is one of two or more different nuclides of the same element.
- They have the same number of protons but different nucleon numbers.
- This is because they have extra neutrons.