What is Nuclear Fission?
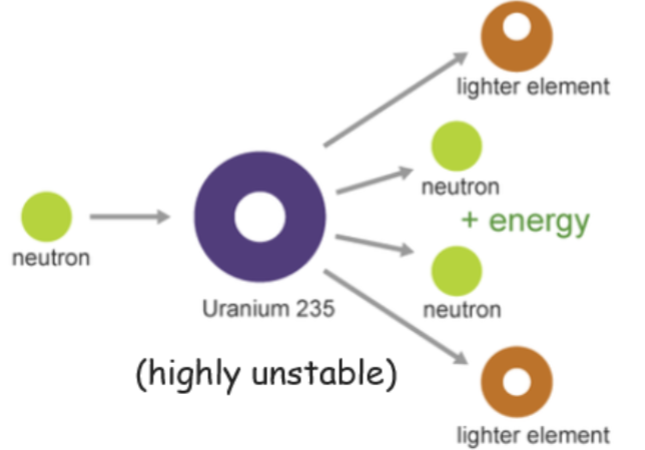
- Nuclear fission is defined as the splitting off a massive nucleus into smaller nuclei.
- It occurs with large nuclei as they are a lot less stable than smaller nuclei, and therefore more susceptible to fission.
- A substance is called fissile if it is capable of sustaining a nuclear fission chain reaction.
- After nuclear fission occurs, the resulting nuclei are called fission fragments.
- Typically, neutrons and gamma rays are also released during the fission.
- After nuclear fission occurs, the resulting nuclei are called fission fragments.
- Typically, neutrons and gamma rays are also released during the fission.
- Nuclear fission can sometimes be a spontaneous (random, without cause) form of radioactive decay of a very massive nuclei.
- The following fission/decay of californium-252 (a nuclide which does not occur naturally) is an example:
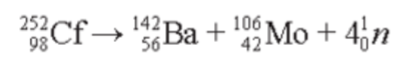
- More important than spontaneous fission, fission can be deliberately induced in nuclei which would not otherwise readily split into fragment.s
- Most commonly, nuclear fission is induced by using slow-moving neutrons.
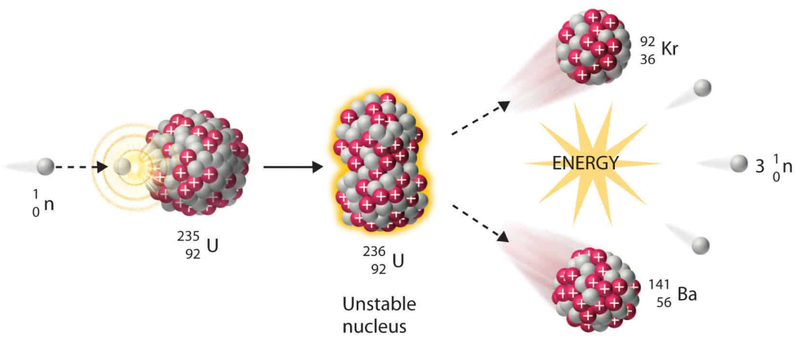
- The majority of the energy released is the form of kinetic energy of the fragments.
- Neutrons and gamma rays also transfer significant energy.
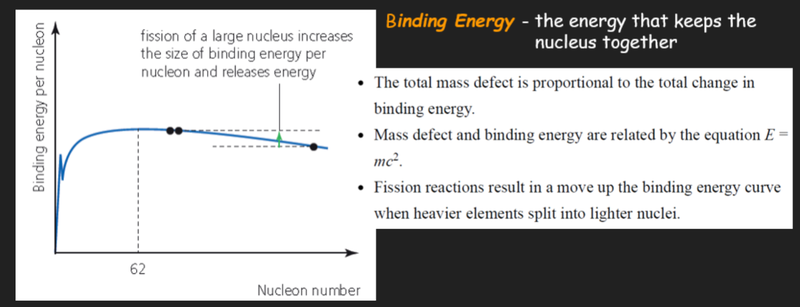
Changes of Binding Energy and Mass
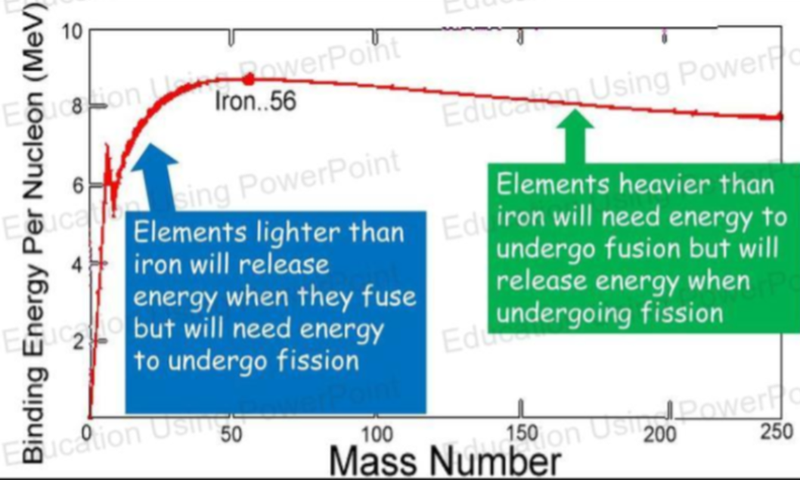
- For nuclear fission to occur, the fission fragments must be more stable than the original nucleus.
- That means that the fission fragments must have higher values of binding energy per nucleon than the original nucleus undergoing fission.
Fission vs Fusion
- Nuclear fission splits a larger atom into 2 or more smaller ones.
- Nuclear fission releases large amounts of energy.
- Energy is transferred from the nuclear potential energy store of a nucleus to the kinetic energy store of the fission fragments.
- Fusion joins 2 or more lighter atoms into a larger one.
- A positive mass defect means that the reaction will release energy.
- Δm = mass of reactants - mass of products
Sources