What is the Oil Drop Experiment?
- Millikan's oil drop experiment was an experiment performed by American physicists Robert A. Millikan and Harvey Fletcher.
- The experiment determined the value of the fundamental elementary charge, qₑ = -1.6 * 10⁻¹⁹ C.
- In the experiment, a setup is created where very tiny oil drops are gathered in a chamber.
- One by one they are given a charge and allowed to drop down into a uniform electric field below.
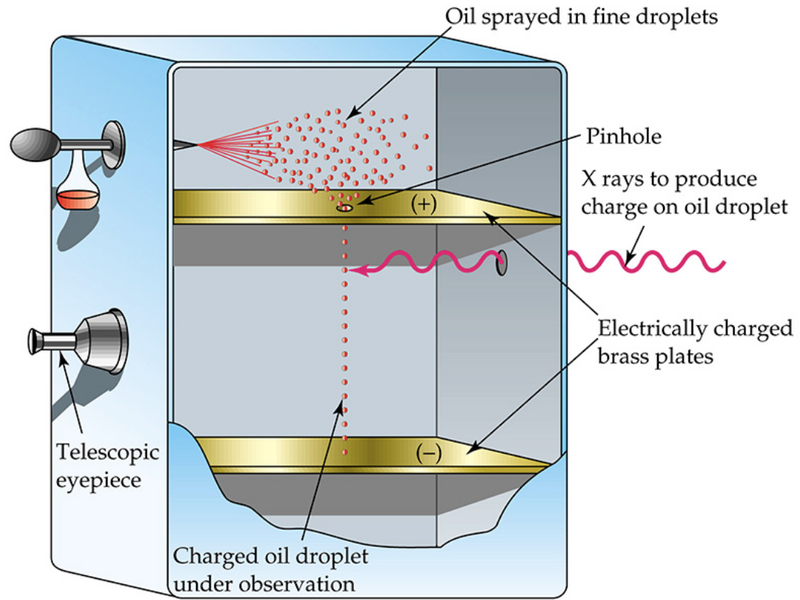
- Eventually the drop then reaches its terminal velocity, which allows us to obtain the mass by equating the gravitational force with the force of the air resistance on the drop.
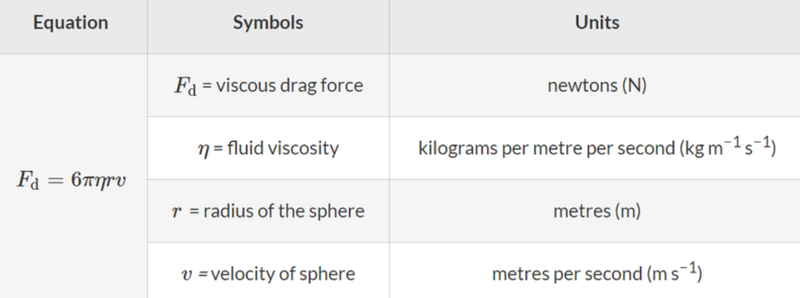
- The viscosity of air is known, and the radius and velocity are known.
- For the gravitational force, Fg = mg, the gravitational acceleration of free fall is known, meaning that the only unknown variable is mass, which can be found by equating Fd = Fg.
- Then, by varying the electric potential difference (voltage) of the plates, the drop was made to stand still.
- The charge of the drop was determined by equating the electric force with the gravitational force on the drop.
- The charges of all the drops were multiples of the same number, -1.6 * 10⁻¹⁹ C.
- This experiment confirmed that electric charge is a quantized quantity.
Sources