What is Entropy?
- Entropy is often something you hear used in bad sci-fi movies, but in actuality it is a physical quantity.
- It is denoted as S, and is a thermodynamic quantity that relates to the degree of disorder of the particles in a system.
- In simpler terms, it is the measure of how spread out or disorderly energy has become.
- Take for example, a heat engine.,
- If the engine was being used to lift up a load we could say that if 100% efficient:
- Internal energy -> Gravitational potential energy
- However, this would never happen as the internal energy of a gas is random and disorderly whereas the gravitational potential energy of the load lifted up would be orderly.
- Thus we can say that:
- If the engine was being used to lift up a load we could say that if 100% efficient:
- It is not possible to increase the amount of order in a system.
Reversible and Irreversible Changes
- Processes can be organized into reversible and irreversible processes.
- A reversible process can be reversed so that the system and all of its surroundings return to their original states and there is no change in entropy.
- Note that there is no external forces acting on the system.
- Reversible processes are an impossibility in the macroscopic world due to phenomena such as friction, fluids mixing, chemical reactions and electrical resistance which all increase entropy.
- Irreversible processes cannot be reversed.
- Entropy always increases within irreversible processes.
- All real macroscopic processes are irreversible.
- If the original state of a system and its surroundings can be restored exactly, the process is described as reversible.
- It can be considered that macroscopic processes in real isolated systems are almost always irreversible and consequently the entropy of real isolated systems always increases.
- Without outside interference, in any process, ordered energy of particles will be irreversibly transferred to disordered energy.
- Take, for example, a bouncing ball.
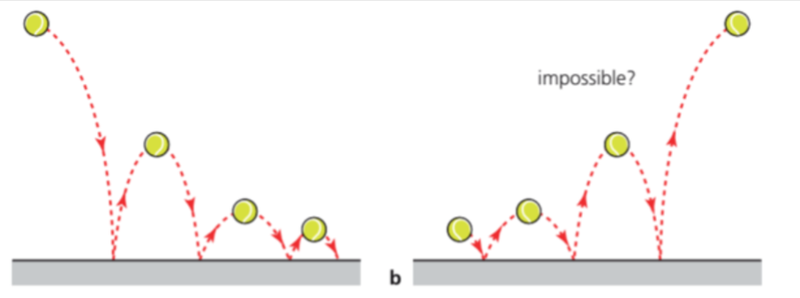
- As the ball bounces, part of its ordered gravitational potential energy is transferred into disorderly energy, such as heat and sound into its surroundings.
- Eventually the ball will come to a stop as all its energy is now disorderly.
- However, if the process were to be reversible, then the ball would be able to bounce back up to its original height, which should be impossible.
The Second Law of Thermodynamics
- The second law of thermodynamics refers to the change in entropy of an isolated system and sets constraints on possible physical processes and the overall evolution of the system.
- It shows that he entropy of a non-isolated system can decrease locally, but this is compensated by an equal or greater increase of the entropy of the surroundings.
- In simpler terms, it states that:
- In any cyclic process, the entropy will either stay the same or increase.
- In simpler terms, it states that:
Examples of Entropy
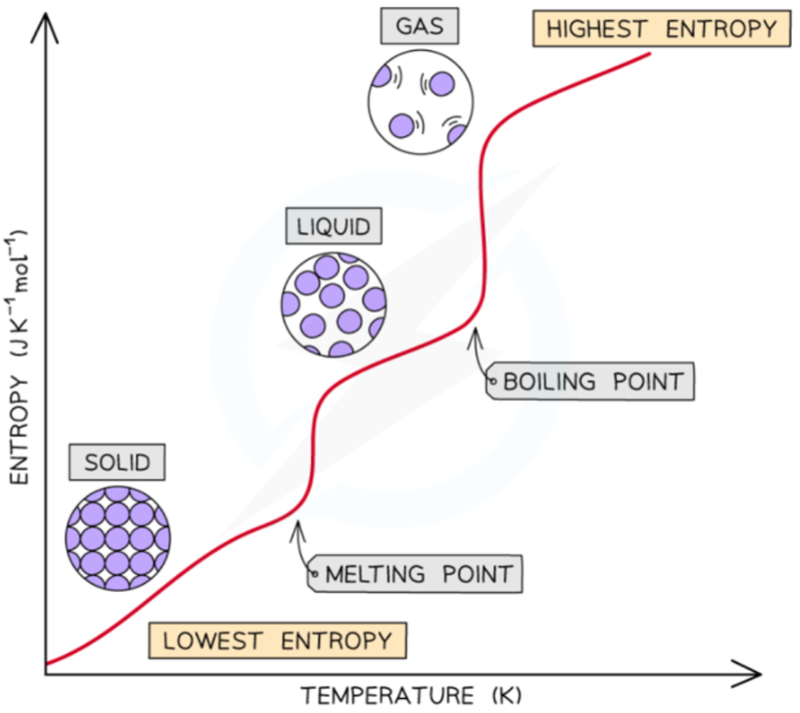
- Gas in a large volume is more disordered (greater entropy) than the same gas at the same temperature in a smaller volume.
- Gas at a higher temperature is more disordered (greater entropy) than the same gas at the same volume at a lower temperature.
- A liquid is more disordered (greater entropy) than a solid of the same material at the same temperature.
- Entropy can also increase when:
- A solid dissolves in a solvent
- A gas diffuses in a container
Entropy and Temperature
- As the temperature of a substance increases, the particles become more spread out and disordered, which means entropy increases.
Clausius Statement of the Second Law of Thermodynamics
- Recall the model for the heat engine.
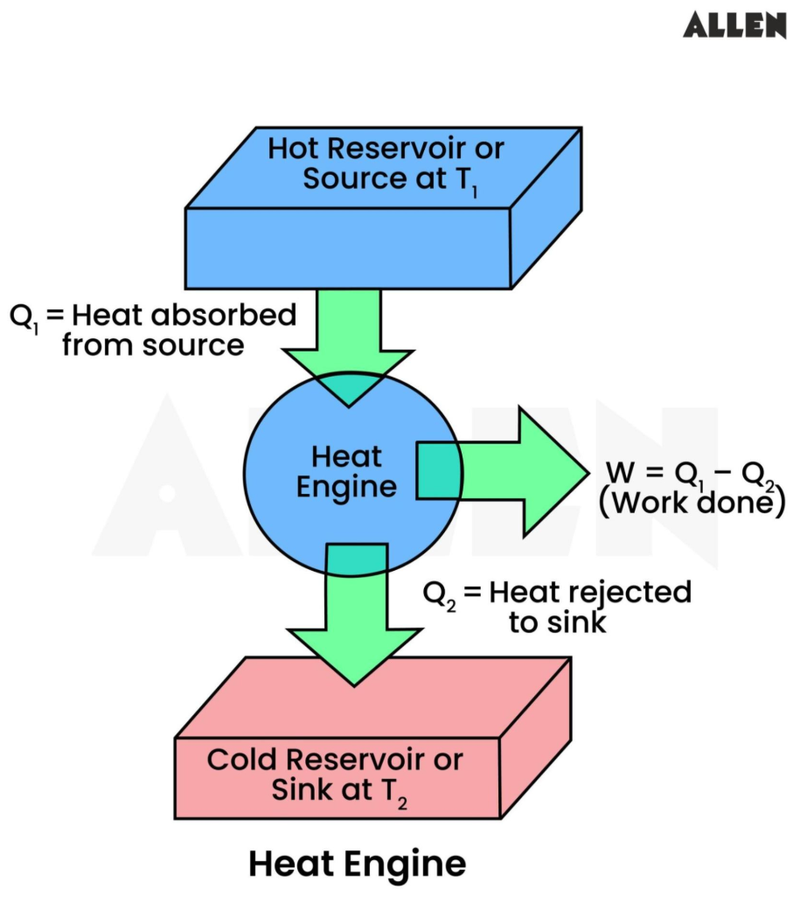
- To make energy go from the cold sink to the hot source we would need to put work in.
- Therefore it is not possible for heat to be transferred from a cold body to a warmer one without work being done.
- However, work doesn't need to be done to transfer heat from a warm body to a cold one.
Kelvin-Planck Statement of the Second Law of Thermodynamics
- Recall the heat engine once more.I
- If the heat engine was being used to lift up a load and it was 100% efficient, then:
- Internal energy -> Gravitational potential energy
- However, if we apply the second law of thermodynamics we see that:
- It is not possible for a heat engine working in a cycle to absorb thermal energy and convert it all to work.
Entropy in Mathematical Terms
- Entropy can be determined in terms of macroscopic quantities such as thermal energy and temperature, as given by the formula:
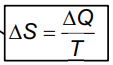
- Where ΔS is the change in entropy.
- ΔQ is the change in thermal energy of the system.
- T is the temperature of the system.
- From this formula we can see that entropy uses the units Joules per Kelvin.
Microstates
- Microstates are the numerous possible combinations of microscopic properties of a thermodynamic system.
- For a two-compartment container which holds N distinguishable particles, the number of microstates in the final volume is:
- Ω = 2ᴺ
- Consider the general case of a system composed of N particles distributed among n boxes.
- The number of possible microstates for such a system is given as:
- Ω = nᴺ
- Take for example, three particles A, B and C, arranged in two boxes.
- The possible arrangements would be:
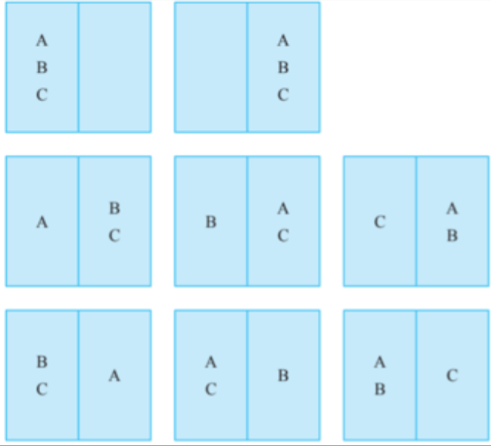
Calculating Entropy of Particles
- Now that we know the definition of microstates, we can also define entropy in terms of the behavior of individual particles.
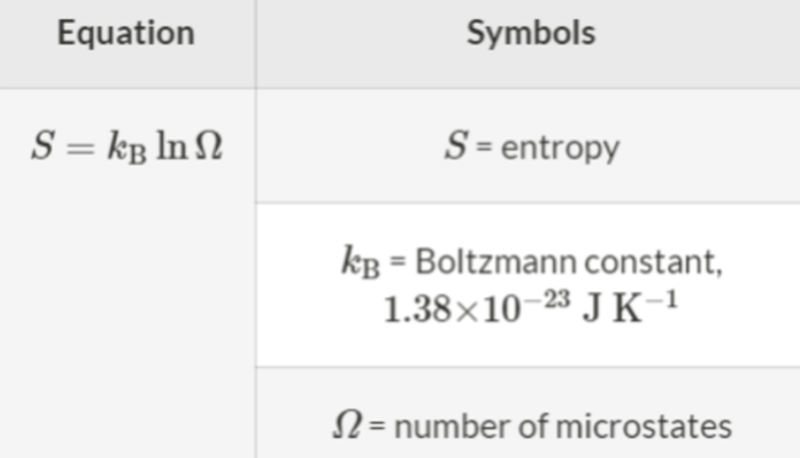
- Likewise, we can also use this formula for a change in the number of possible microstates.
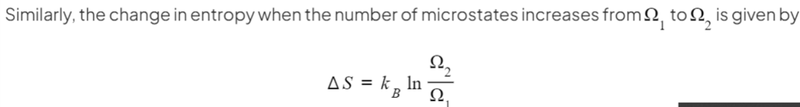
- This formula is derived from the previous one using logarithm rules.
Sources