Efficiency
- When work is done, energy is always transformed into different forms of energy.
- Efficiency is the measure of how much useful energy you get out of a process from the energy you put into it.
- For example if you were to lift a box, you would transfer chemical energy into gravitational potential energy and thermal energy (heat from your mitochondria burning fuel).
- Thermal energy in this scenario is not wanted and the energy converted to heat is counted as an inefficiency.
- 100% efficiency is impossible as it would lead to perpetual motion.
- Imagine for example if you spun a cog, and it kept spinning forever.
- This doesn't happen in reality as the cog continuously loses kinetic energy as it is transferred into thermal energy and sound.
Calculating Efficiency
- Calculating efficiency is quite simple, as it is simply a ratio of useful energy outputted and the amount of energy inputted into a system.
- Power can be used as a substitute for energy, as power is defined by E/t, when power is divided by power, the t cancels out, leaving the ratio of energy.

- As efficiency is a ratio, it has no units.
- And as nothing is 100% efficient, the value obtained for efficiency can never be greater than 1.
- It will always be a decimal number equal to or greater than zero, but less than one.
- If converted to a percentage, efficiency should always be greater than or equal to 0% but less than 100%.
Sankey Diagrams
- Sankey diagrams can be used to show where energy is transferred during a process.
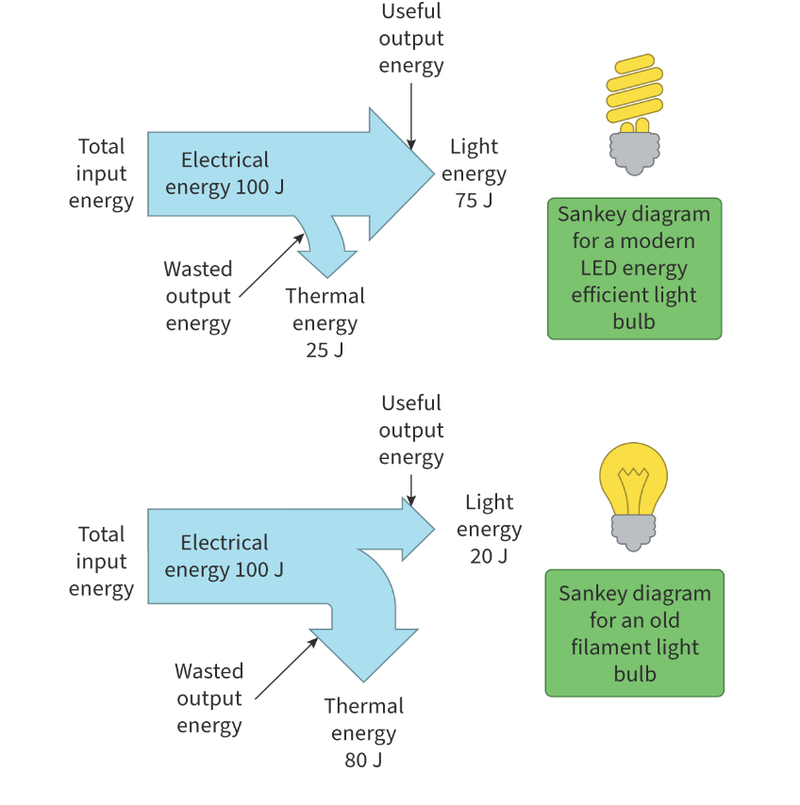
- The arrows are sized based on the amount of energy that is transformed into the specified type.
- For example, in the first diagram, the arrow that shows light energy is 3 times wider than the arrow that shows energy dissipated as thermal energy, as 75J is converted into light and 25J into heat.

