Batteries
- Batteries, or cells, provide electromotive force in a circuit.
- There are various different types of batteries, and they can be found in many places, such as your phone, car or calculator!
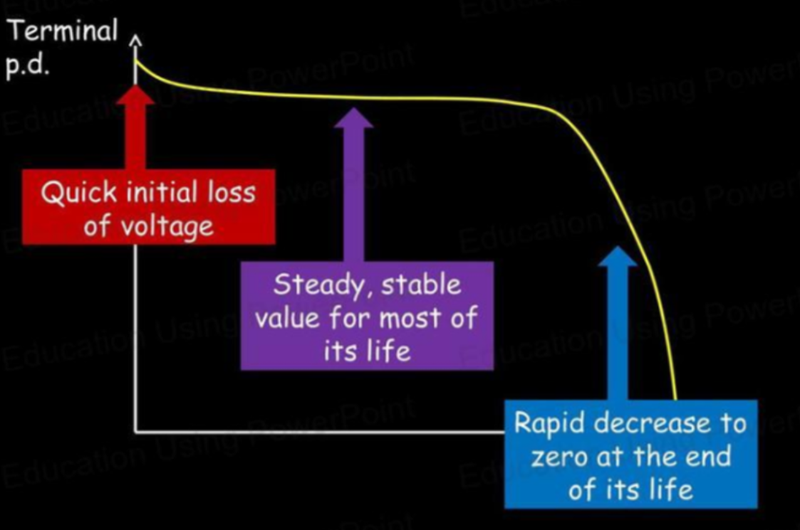
How Cells Lose Voltage Over Time
- Has your battery ever "run out of juice"?
- This is because the cell can no longer provide electromotive force due to degrading over use.
- The degradation isn't a steady decline but instead is quicker at the start and end of a battery's life cycle.
Recharging a Battery
- Luckily, you don't have to replace your phone every time it runs out of battery (that would suck), this is because it can be recharged!
- By hooking up a battery to another battery or other power source, you can recharge it, and it will regain the ability to provide electromotive force!
- The positive terminal of the battery should be connected to the positive terminal of the recharging device and the negative terminals of the battery should likewise be connected to the negative terminal of the recharging device.
- A primary cell can't be recharged.
- A secondary cell can be recharged by applying an external voltage, reversing the chemical reaction that occurs in a cell.
Cells
Chemical Cells
- Most batteries are chemical cells which use volatile metals such as lithium.

- Although they very dangerous to make and handle, simple, albeit weaker, batteries can be made with simple supplies, using the same principles as those batteries.
- To make a chemical cell, or a battery, you can begin with a container of weak acid and two electrodes made of different materials.
- Different metals dissolve in acids at different rates.
- When a metal dissolves, it enters the acid as a positive ion, leaving behind an electron.
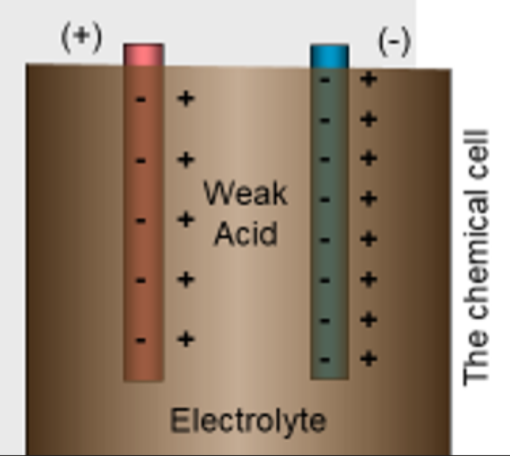
- We call the weak acid the electrolyte.
- We call the negative metal the positive terminal.
- We call the most negative metal the negative terminal.
- Each time an electron leaves, the negative terminal, the acid creates another electron.
- Each time an electron enters the positive terminal, the acid neutralizes an electron.
- This process is maintained until one of the metals or the electrolyte is completely used up.
Solar Cells
- A solar cell is the photovoltaic device that converts light energy (which comes from the sun) into electrical energy.
- This device works on the principle of the photovoltaic effect.
- The photovoltaic effect is the photogeneration of charge carriers in a light absorbing materials as a result of light radiation.
- N-type semiconductors are doped with elements that have more valence electrons (phosphorus, arsenic).
- P-type semiconductors are doped with elements that have fewer valence electrons (boron, gallium).
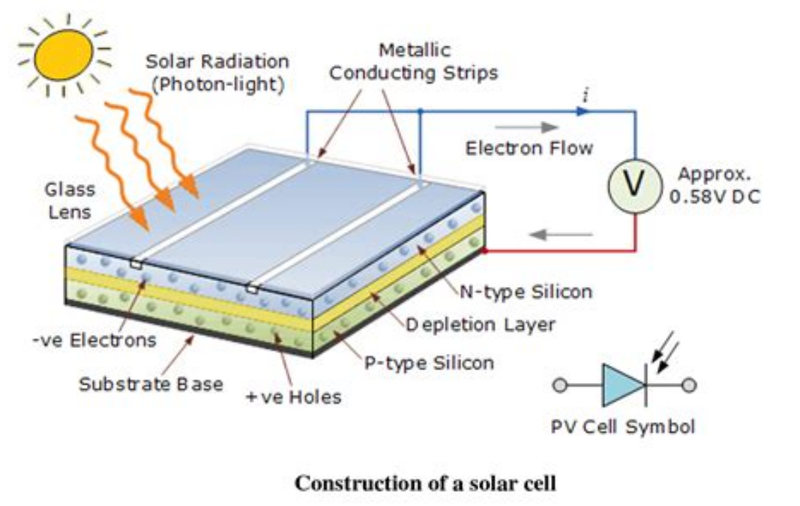
Construction of Solar Cells
- Solar cells consist of an n-type semiconductor, called an emitter and a p-type semiconductor layer called a base.
- The two layers are sandwiched and hence the formation is a p-n junction.
- The surface is coated with anti-reflection coating to avoid the loss of incident light energy due to reflection.
- Proper metal contacts are made on on the n-type and p-type side of the semiconductor for electrical connection.
Working of a Solar Cell
- When a solar panel is exposed to sunlight, the light energies are absorbed by the semiconductor materials.
- Due to this absorbed energy, the electrons are liberated and produce the external DC current.
- The DC current is converted into a 240-volt AC current using an inverter for different applications.
Sources
https://repairmachinemazurskimcl.z14.web.core.windows.net/lithium-battery-12-volt-100ah.html
https://manuallaneirwg1bq.z21.web.core.windows.net/single-solar-cell-diagram.html