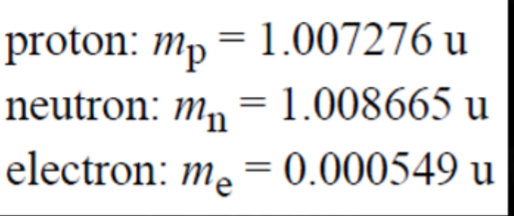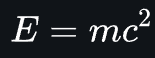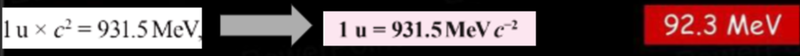Unified Atomic Mass Unit
- The unified atomic mass unit, u, is used to measure the mass of atoms relative to one another.
- The mass of a single carbon atom is approximate 1.99*10^-26kg.
- As carbon-12 has 12 nucleons, 1 nucleon must be 1/12th of that mass.

- According to a periodic table, the relative atomic mass of hydrogen is 1.0079.
- This means its mass is m = 1.0079u, or 1.67*10^-27kg.
Binding Energy
- Consider a carbon-12 atom again.
- It has 6 protons, 6 neutrons and 6 electrons.
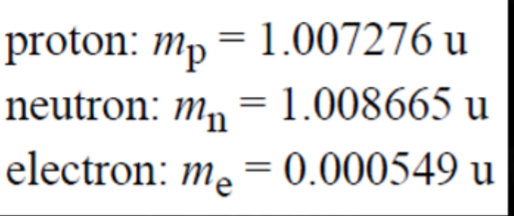
- Clearly a carbon-12 atom has a mass of 12u.
- The actual mass of the carbon nucleus can be calculated as:

- However, if we add up the mass of 6 neutrons and 6 protons, we get something different!
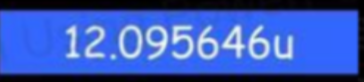
- This is strange! There's a difference between the defined mass using the unified atomic mass unit and the measured mass by adding up protons and neutrons.

- Has physics failed us? Not quite!
- This difference is called the mass deficit and it exists because some of the mass is in the form of energy that keeps the nucleus together.
- This is called the binding energy of the nucleus.
- It can be alternatively defined as:
- The amount of energy needed to completely separate the nucleons.
- The energy released when a nucleus is formed from nucleons.
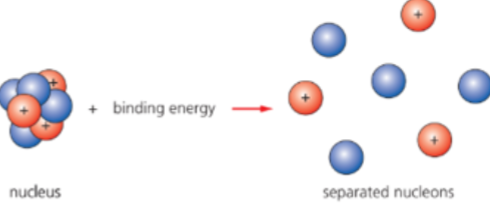
- The binding energy BE is measured in the megaelectronvolt, MeV.
- Binding energy increases as the number of nucleons in the nucleus increases, because it requires more energy to separate more nucleons.
Energy-Mass Equivalence
- Energy being in the form of mass might be strange, but it brings us to the most famous formula in physics:
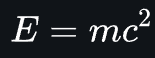
- This equation shows that mass is just another form of energy.
- While speculated on before several times, Albert Einstein was the first to properly deduce this formula.

- It can also be given in the form of atomic mass units.
- If an object gains energy its mass increases ever so slightly.
- However, the change is far too small to detect.
- Mass is the energy an object has because of the matter it is made of.
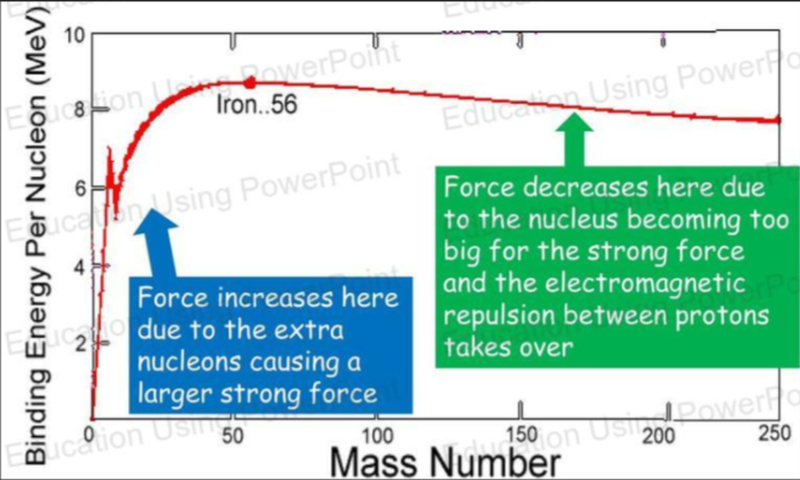
Binding Energy Per Nucleon
- Using the energy-mass equivalence formula, and the mass of the binding energy for carbon-12, we can work out that each carbon-12 nucleus has a binding energy of 1.48*10^-11J.
- Calculating this in eV gives us:
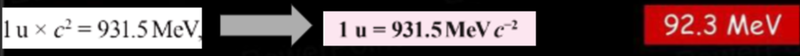
- The binding energy per nucleon is calculated as 7.68MeV.
- That means we can calculate binding energy per nucleon as:

- The A here is the atomic number, not to be confused with activity.
- Binding energy per nucleon tells us how strongly each nucleon is held together.
The Energy Inside a Nucleus
- How is the mass defect of a nucleus related to its binding energy?
- Consider nucleus B with a large mass defect:
- The mass of the nucleus is much less than the mass of its separated nucleons.
- The energy of the nucleus is much less than the energy of its separated nucleons (since mass is energy.
- To separate the nucleus into its nucleons, the amount of energy in B would need to be supplied as work.
- The nucleus has a large binding energy (equal to the energy in B).
- The nucleus is difficult to separate into its individual nucleons.
- This difficulty in separating nucleons means it is more stable.