What are Atomic Energy Levels?
- In an atom, electrons orbit the nucleus.
- Electrons cannot be at any distance from the nucleus.
- They can orbit at discrete distances from the nucleus.
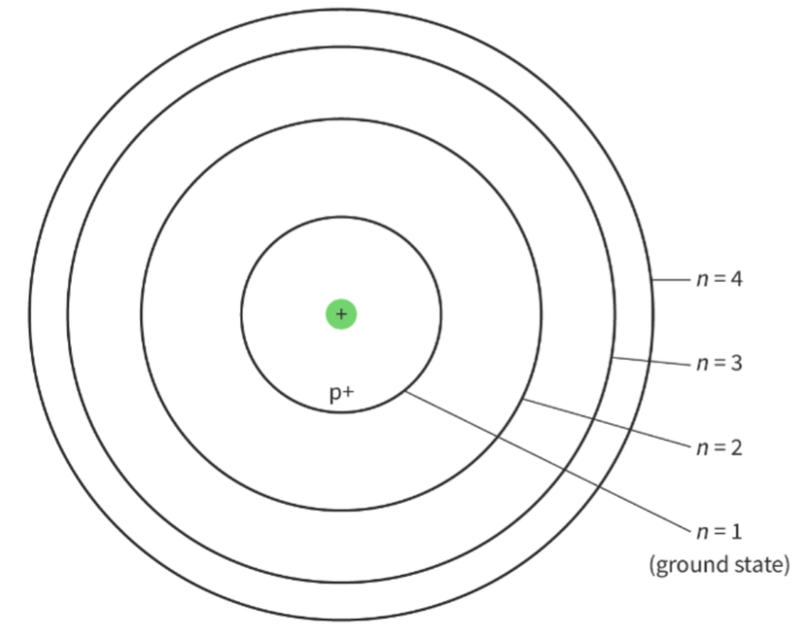
- Therefore, atoms have a set of discrete energy levels that correspond to the allowed orbits of the electrons.
- We can assign an integer value n to every level.
Evidence for Energy Levels within Atoms
- By looking at the light emitted by an excited sample of atoms we can determine energy levels by the frequencies of light emitted.
- Likewise, if we shine light though an absorbing gas, we can see that they absorb the same frequencies they would emit when given energy.
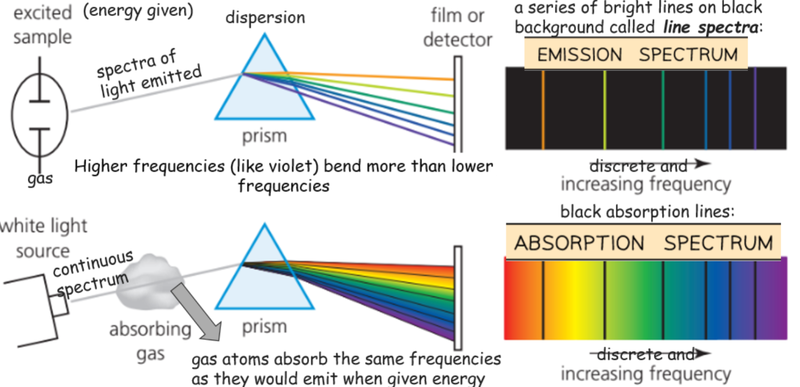
- Each line on an absorption spectrum is explain by the electrons moving to a higher energy level in an atom.
- Each line on an emission spectrum is explained by electrons moving to a lower energy level within the atom.
- Each line corresponds to a precise frequency.
Understanding Energy Levels
- An atomic energy level is one of a series of possible discrete and separate energy levels of an electron within an atom.
- The ground state is the lowest energy state of an atom or electron.
- Ionization energy is the amount of energy needed to remove an electron from an atom or molecule.
- It is the energy needed to make the energy level = 0
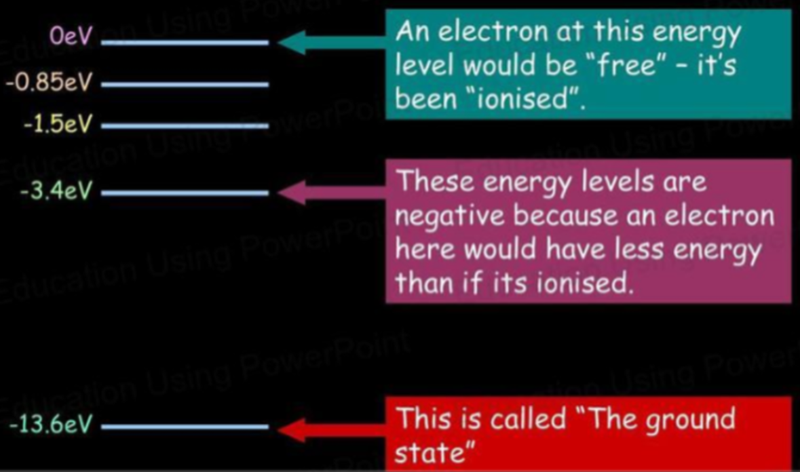
- The energies of electrons within atoms are given negative values because we would have to supply energy to remove them from the atom (to infinity), where they would then be considered to have zero electric potential energy.
- This is similar to gravitational potential energy, where you need to supply energy to increase an objects GPE (less negative), so that there is more potential for gravity to do work.
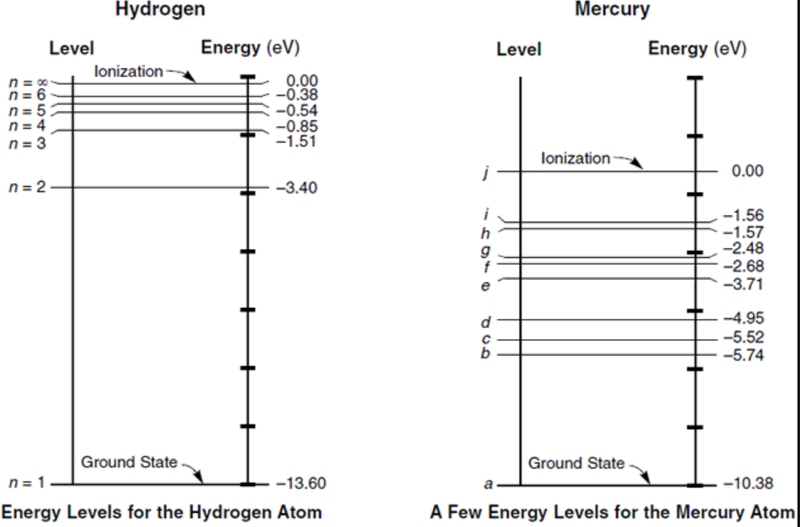
- The highest energy level shown is equivalent to removing the electron from the atom (to infinity, where E = 0).
- That is, the ionization energy of hydrogen atoms is 13.6eV.
- It is different for every different element.
- An atom changes its energy level when electrons change their distance from the nucleus.
- This change in distance happens in a discrete way.
- The electrons "jump" from one orbit to another.
- This is called atomic transition.
- This transition from a lower energy level to a higher energy level is called excitation.
- The transition from a higher energy level to a lower energy level is called de-excitation.
- When an atom gains energy, an electron may transition to a higher energy level.
- When an electron loses energy, it transitions to a lower energy level.
- The atomic energy levels are discrete, so the energy emitted or absorbed is also discrete.
- These packets of electromagnetic energy are called photons.
- You might know them as light!
- The transition from a higher energy level to a lower energy level is called de-excitation.
The Photon Model
- Photons are fundamental particles that make up all forms of electromagnetic radiation.
- A photon is defined as a massless "packet" or a "quantum" of electromagnetic energy.
- Therefore energy transferred by a photon is not continuous but as discrete packets of energy.
- Each photon carries a specific amount of energy and transfers this energy all in one go.
- During the transition between energy levels a photon is emitted when an atom makes a transition to a lower energy level.
- The energy of the photon is equal to the difference in the energy of the levels involved.
Calculating Photon Energy
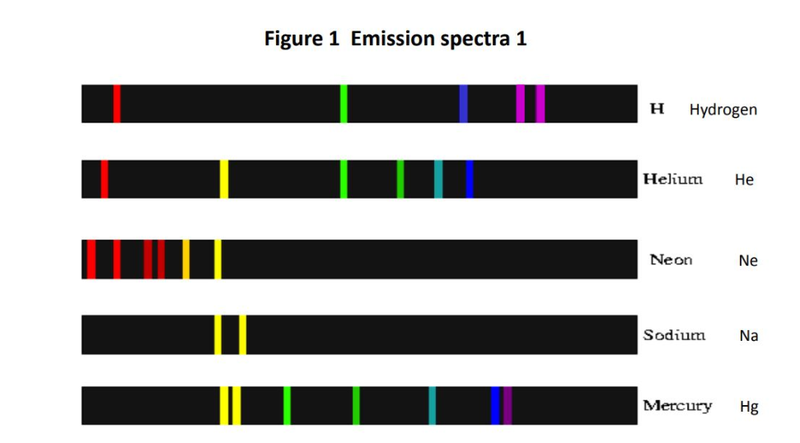
- Spectral lines are used to determine atomic energy levels.
- The difference between the energy levels is equal to the energy of the photon released.
- E = energy of the photon (in Joules)
- The difference between the energy levels is equal to the energy of the photon released.
- We can find the energy using the properties of the photon as well, using the equation:
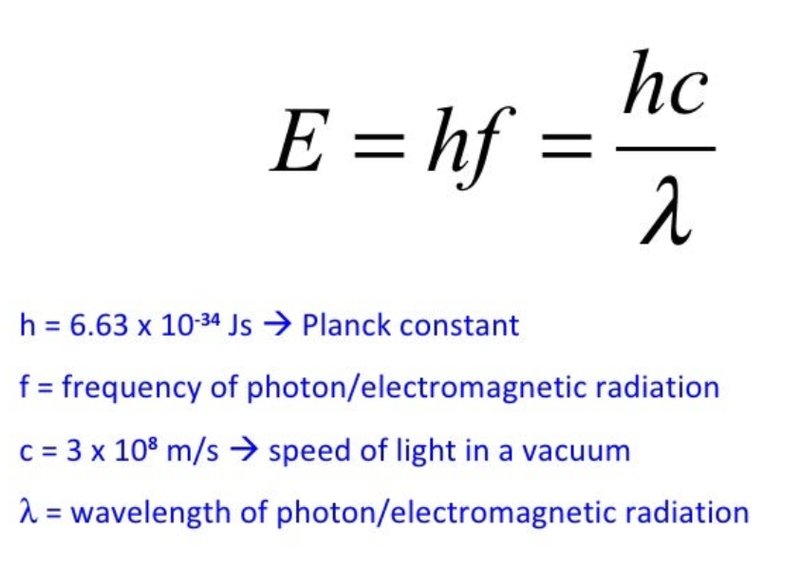
- Planck's constant is a fundamental constant of quantum physics which connects the energy and frequency of a photon.
Sources
https://www.slideshare.net/slideshow/photon-and-energy-levels/9432668