Moles
- While mass can be used to measure the amount of a substance, it is difficult to compare as mass varies a lot between different substances due to different densities.
- Instead, moles are used to measure the amount of substance by giving the amount of particles.
- A mole of a substance always contains the same number of molecules, ions, particles or atoms.
- This amount is called Avogadro's constant.

- The number of moles in a substance can be found by dividing the number of particles by the number of moles.
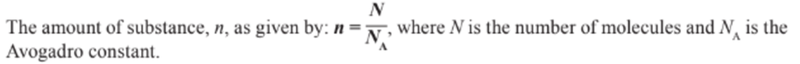
Molar Mass
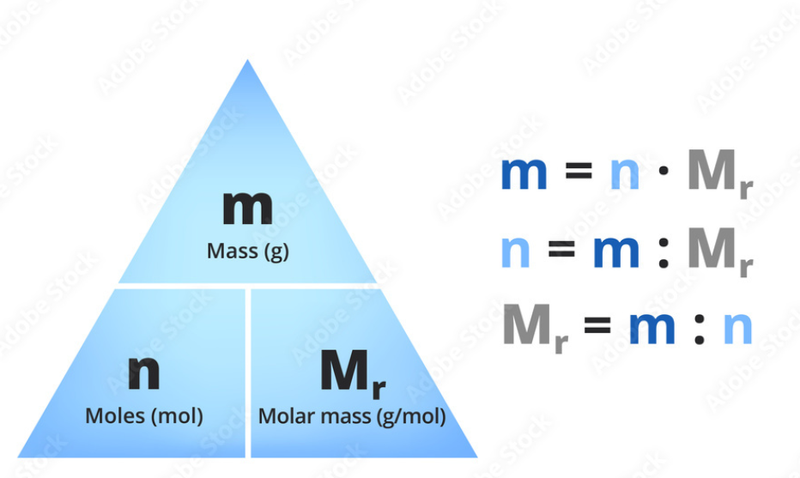
- Molar mass is the mass of one mole of a substance.
- Molar mass varies between substances.
- For example helium has a smaller molar mass than titanium.
- The relationship between molar mass, mass and the number of moles is as follows:
Sources