Electron Structures
- The periodic table can describe the electron arrangement of any element.
- An atom consists of a nucleus and a cloud of electrons.
- Protons and neutrons are part of the nucleus, and they are referred to as nucleons.
- Atoms in a normal state have an equal number of protons and electrons, thus remaining neutrally charged.
- They also usually have the same number of neutrons, however isotopes of the element can have different numbers of neutrons.
- Thus the periodic table can show how many protons and electrons an atom has, this is called its atomic number.
- Groups are the vertical rows of the periodic table and indicate how many valence electrons an atom has.
- Periods are the horizontal rows which indicate the amount of electron shells an element has.
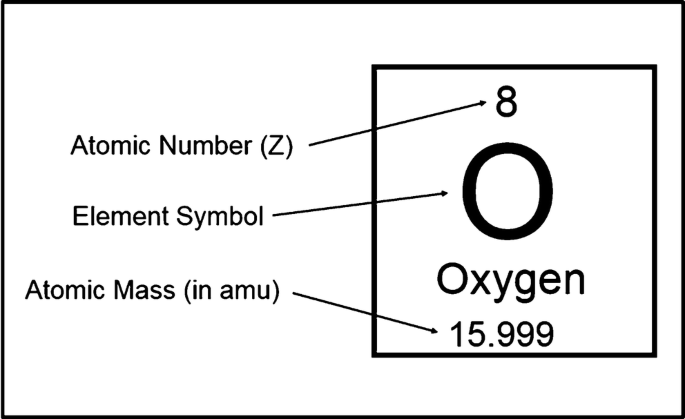
- This what Oxygen would look like on the periodic table. We can infer from its atomic number that it has 8 electrons, 8 protons and 8 neutrons (unless it is an isotope).
- The atomic mass number shows the amount of particles an atom has in its nucleus, which in this case rounds to 16 (8 protons and 8 neutrons).
Chemical Elements
- Every atom of the same element has the same number of protons.
- The number of protons determines what element an atom is.
Isotopes
- Isotopes are atoms with the same number of protons but a different number of neutrons.
- This works because neutrons don't have an electrical charge so the atom still has a neutral charge.
Examples
- Protium is the standard hydrogen isotope, having 1 proton and making up 99.985% of all hydrogen.
- Deuterium is an isotope of hydrogen with 1 proton and 1 neutron. They represent 0.015% of all hydrogen.
- Tritium is an isotope of hydrogen with 1 proton and 2 neutrons. They are unstable and radioactive so they decay in nature.
- Carbon 12 is the most normal form of carbon, with 6 protons and 6 neutrons.
- Carbon 13 is a less common form of carbon with 7 neutrons instead of 6.
- Carbon 14 is an unstable form of carbon, and thus it is radioactive, slowly decaying over time. Its half life is 5730 years, meaning that in 5730 years, Carbon 14 will have lost half of its mass.
Modeling Electron Shells
- The Bohr model is a simple way to describe how the electrons circulate the nucleus of the atom on circular orbits.
- Silicon has 14 electrons for example, and its electron configuration is 2 on the first ring, 8 on the second ring, and the remaining 4 on the outer ring.
- The rule for the amount of electrons on each shell is 2n².
- So the first shell would have 2 electrons, the second 8, the third 18, the fourth 13 and so on.
- Atoms don't usually fully fill up all electron shells so it is more reliable to use the periodic table to determine the amount of electron shells an atom has, especially for larger atoms.
- This same arrangement can also be illustrated through the Lewis, or dot model.
- The Lewis model can be more useful for illustrating atomic bonds, as it only draws the valence electrons of an atom.
Subshells
- The best way to model the arrangement of electrons is a quantum mechanic atomic model.
- In this model, the electrons are delocalized.
- This means that they aren't the exact location of the electron but simply where the electron could be during any point of time.
- They are called orbitals and are round, three-dimensional shapes.
- Each orbital can only have two electrons with different spins.
- Orbitals form sets that have a similar and symmetrical shapes.
- A subshell (s, p, d and f) is composed by orbitals.
- Orbitals in the same subshell have the same energy.
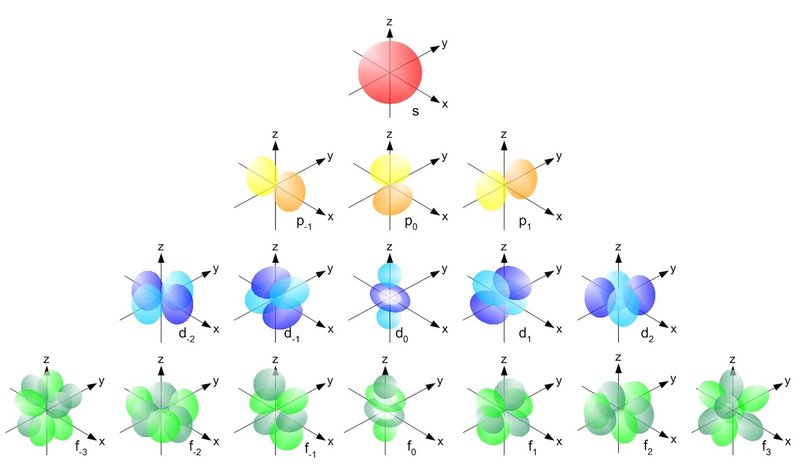
- A picture showing the shapes of different types of subshells.
- Notice that one "sphere" represents an orbital of 2 electrons.
Rules to Follow
- Paul's Exclusion Principle states that no more than two electrons can occupy the same orbital and two electrons in the same orbital must have opposite spins.
- Hund's rule: "Every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied and all electrons in singly occupied orbitals have the same spin."
- This means that subshells can either be fully-filled, or filled-halfway.
- For example, Copper has 29 electrons, and thus if organized into the subshells in the same order as in other elements, the subshell 3d ends up missing 1 electron from being full.
- That's why instead of leaving 1 missing electron in the subshell 3d (which can contain 10 electrons) we leave 1 empty electron in the subshell 4s prior to it (which can only contain 2 electrons), thus making it half full.
Different Types of Subshells
- Different subshells can contain different amounts of orbitals and thus electrons.
- Subshell S has 2 electrons.
- Subshell P has 6 electrons.
- Subshell D has 10 electrons.
- Subshell F has 14 electrons.
Aufbau Principle
- The Aufbau Principle states that "The orbitals with lower energies are filled before those with higher energies."
- This means that the lower energy orbitals are practically always occupied, however higher energy ones can only be occupied by atoms with more electrons.
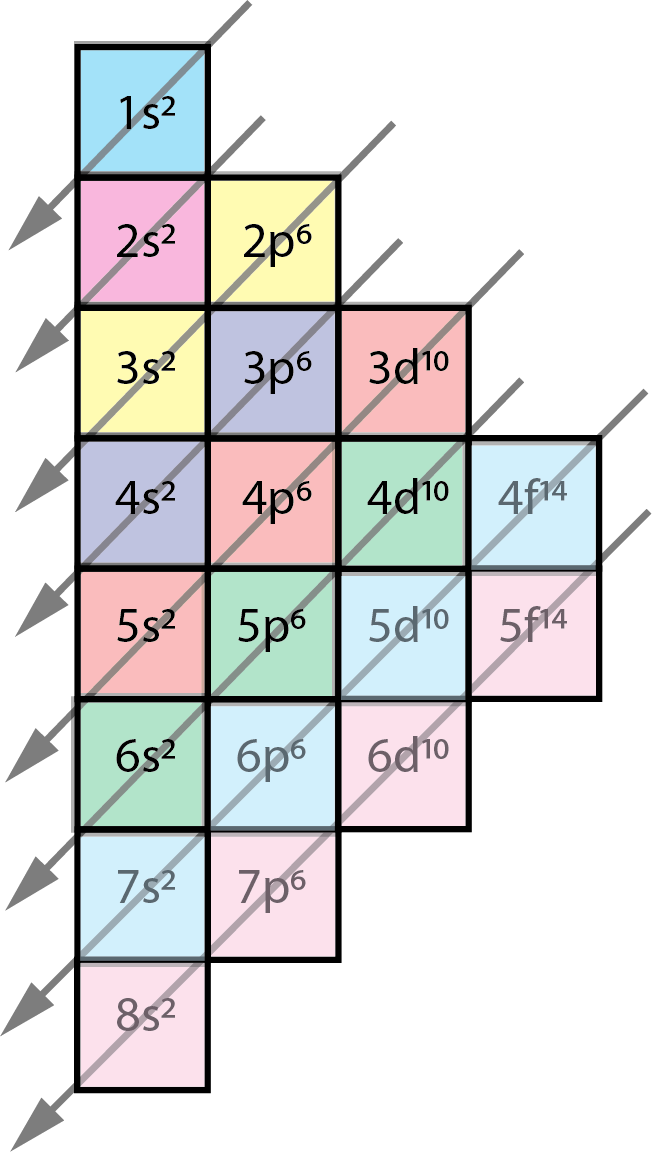
- The order follows the arrows.
- The number in front shows the horizontal row of the subshell, and the superscript annotates the number of electrons in the orbital.
- When filling an atom you'd start with subshell 1s, then 2s, then 2p, 3s, 3p, 4s and so on.
Image Sources
https://link.springer.com/referenceworkentry/10.1007/978-3-319-39312-4_244
https://peda.net/joensuu/lukiot/lyseon-lukio/oppiaineet2/kemia/kemia-2/2oyrjo/2ksmh